Chromoblastomycosis is a chronic, granulomatous, suppurative mycosis of the skin and subcutaneous tissue caused by traumatic inoculation of dematiaceous fungi of the family Herpotrichiellaceae. The species Fonsecaea pedrosoi and Cladophialophora carrionii are prevalent in regions where the disease is endemic. Chromoblastomycosis lesions are polymorphous: verrucous, nodular, tumoral, plaque-like, and atrophic. It is an occupational disease that predominates in tropical and subtropical regions, but there have been several reports of cases in temperate regions. The disease mainly affects current or former farm workers, mostly males, and often leaving disabling sequelae. This mycosis is still a therapeutic challenge due to frequent recurrence of lesions. Patients with extensive lesions require a combination of pharmacological and physical therapies. The article provides an update of epidemiological, clinical, diagnostic, and therapeutic features.
Chromoblastomycosis (CBM) is a chronic, granulomatous mycosis of the skin and subcutaneous tissue produced by the traumatic inoculation of various dematiaceous fungi of the order Chaetothyriales and family Herpotrichiellaceae, present in soil, plants, and decomposing wood, prevalent in tropical and subtropical regions of the world.1,2 CBM is a progressive, disabling, difficult-to-treat occupational disease, evolving with episodes of secondary bacterial infections, leading to low work productivity and frequent absenteeism. The synonyms for this mycosis vary widely, including: chromomycosis, verrucous dermatitis, Lane-Pedroso’s mycosis, Fonseca’s disease, Carrión’s mycosis, cladosporiosis, figueira, formigueiro, blastomycosis nigra, sunda, susna, and chapa, among others.
HistoryIn 1922, Terra et al. coined the term chromoblastomycosis to refer to the disease.3 Seventy years later, in 1992, the term was proposed by the International Society for Human and Animal Mycology (ISHAM) as the official name for the mycosis from that publication forward.4 McGinnis, in 1983, finalized the long controversy over the nomenclature for the mycosis with the publication in which he clearly established the concept of chromoblastomycosis, differentiating it from phaeohyphomycosis and other infections caused by fungi of the family Herpotrichiellaceae (order Chaetothyriales).1 CBM is currently classified by the International Classification of Diseases as follows: ICD-9 117.2 and ICD 10-B43.5
The first cases of CBM were observed by Pedroso and Gomes in 1911, but it was not until 1920 that the authors published the four cases which they reported as having been caused by Phialophora verrucosa.6 However, Brumpt7 contended that the fungus belonged to a different species, which he named Hormodendrum pedrosoi, later renamed Fonsecaea pedrosoi by Negroni.8 According to Castro and Castro9, the first author to publish was a German physician Max Rudolph10, who lived in Brazil, and who in 1914 published six cases of CBM observed in the town of Estrela do Sul, Minas Gerais State. Rudolph emphasized the disease’s clinical characteristics, and in four of the six cases he cultured and isolated a brownish-black fungus which he inoculated in animals. There is no record of a histopathology report. In 1915, Medlar11 and Lane12 described the first cases of CBM in the United States. Thaxter isolated and classified the fungus from these cases, calling it Phialophora verrucosa.
In 1928, Hoffman reported ten cases of a disease similar to CBM observed by Guiteras in Cuba in 1908, but not published.13 The first case outside of the Americas was described by Montpellier and Catanei in 1927 in an Algerian patient.14 The second case in the United States was reported by Wilson et al. in 1933.15 In 1935, as the name “chromoblastomycosis” suggested that the etiological agents display budding yeasts in the tissue, Moore and Almeida (1935) proposed the term “chromomycosis” to replace “chromoblastomycosis”.16 More cases were reported in European countries.17 The fungus Acrotheca aquaspersa, later Rhinocladiella aquaspersa, was described in 1972 by Borelli.18
The World Health Organization (WHO) keeps a long list of neglected diseases, which is defined as endemic tropical and subtropical diseases in low-income populations that cause thousands of deaths a year. The list includes diseases caused by infectious and parasitic agents (fungi, viruses, bacteria, protozoans, and helminths). In Brazil, the neglected diseases include deep mycoses such as CMB, paracoccidioidomycosis, Jorge Lobo’s disease, mycetomas, sporotrichosis, and others.5
EtiologyThe etiological agents of CBM belong to the order Chaetothyriales, family Herpotrichiellaceae, and include: Fonsecaea pedrosoi, Fonsecaea monophora, Cladophialophora carrionii, Fonsecaea nubica, Phialophora verrucosa, Fonsecaea pugnacius, Rhinocladiella aquaspersa, Cladophialophora samoensis, Cyphellophora ludoviensis, Rhinocladiella tropicalis, and Rhinocladiella similis.1,5,11,12,18-25 Studies on the ribosomal DNA (rDNA) internal transcribed spacer showed that Fonsecaea pedrosoi and Fonsecaea compacta are identical species.26
The most prevalent species (90%) is F. pedrosoi.19,20,27-30 Cases of CBM caused by Exophiala jeanselmei and Exophiala spinifera have been reported in the literature.31-34 In Panama (2007), there is a report of CBM caused by Chaetomium funicola.35
In the tissues, the fungi display a micromorphology of round/oval, brownish, thick-walled cells 4-12 microns in diameter, which multiply by septation in two distinct planes, called muriform (sclerotic) bodies (cells) or Medlar’s bodies, representing the invasive form. The term muriform is preferred to sclerotic, according to Matsumoto.36 The melanin from the dematiaceous fungi is formed by the polymer dihydroxy naphthalene (DHN), which forms the melanin complex by interacting with proteins, lipids, and carbohydrates from the cell wall and represents an important factor in the virulence of these fungi.37
EpidemiologyCBM is a cosmopolitan disease, with the highest prevalence in tropical and subtropical regions between 30° latitude North and 30° latitude South.38 The largest focus of CBM in the world is in Madagascar, Africa.39 However, the mycosis displays variable incidence in South America, Central America, North America, Asia, and Europe. Among the countries with temperate climates, there have been reports in Russia, Canada, Finland, Czech Republic, Romania, and Poland, in addition to high incidence in Japan.17,40-45
In Venezuela, C. carrionii predominates in the arid states of Lara and Falcón, while F. pedrosoi predominates in humid areas.20,23,27,28,46 The mycosis occurs in most states of Brazil, the country with the second largest case series, and where the state of Pará has the highest prevalence.23,28,30,47,48 Current or former agricultural workers, miners, and woodsmen, predominantly males 20 to 60 years of age, account for 90% of the cases. There is no ethnic predilection. In Japan, the lesions predominate on the upper limbs, face, and neck.44,45 There is no record of direct human-to-human or animal-to-human transmission.20
PathogenesisThe etiological agents of this mycosis, generally with low pathogenic power, live as saprophytes in the soil, plants, and organic matter in decomposition. Connant in 1937 demonstrated for the first time that the agents of CBM exist in nature, by isolating fungus Cadophora Americana (later renamed P. verrucosa) from wood.49 Various subsequent studies confirmed the etiological agents’ presence in the environment.50-54 CBM results from the transcutaneous, traumatic inoculation of propagules from various species of dematiaceous fungi. In the host, the propagules adapt to the tissue environment through the dimorphism of the filamentous phase in globe-shaped structures called muriform cells.
The immune response in CBM is not totally clear, although the main response is cellular, involving macrophages, Langerhans cells, factor XIIIa+ dermal dendrocytes, in addition to the humoral response. In 2003, D’Ávila et al. analyzed CBM-spectrum disease, relating the clinical forms to the cytokine profile.55 Verrucous lesions presented parasite-rich granulomas and predominance of IL4 and IL10, a Th2 response. In the atrophic forms, they observed well-formed granulomas with more epithelioid and Langhans cells, IFN-gamma, and TNF-alpha, a Th1 response profile.
Souza et al. (2008) observed that the monocytes of patients with a severe form of the disease showed increased production of IL-10 and lower expression of HLA-DR and costimulatory molecules.56 According to the authors, immune modulation with recombinant IL-12 or anti-IL10 can restore the Th1 immune response in these patients.56
Some studies have addressed macrophage activation and destruction of F. pedrosoi, but there is also in vitro evidence that the fungus can reduce the efficacy of macrophages, with inhibition of the immune response and fungal persistence in the tissues.57-59
Sotto et al. investigated the cellular immune response, especially antigen distribution in patients’ biopsy specimens.60 In their study, the majority of antigens were observed in the cytoplasm of the macrophages, and to a lesser extent in the Langerhans cells and factor XIIIa- positive dendrocytes.60
Gimenes et al. demonstrated that patients with the severe form of CBM produce high levels of IL-10 and low levels of IFN-γ, together with inefficient T-cell proliferation.61 Meanwhile, patients with the mild form show intense production of IFN-γ, low levels of IL-10, and efficient T-cell proliferation. The interaction of conidia or sclerotic F. pedrosoi cells with Langerhans cells with decreased expression of CD40 and B7-2 and immune function inhibition was demonstrated by Silva et al.62 The immunohistochemical analysis of 23 biopsies from the untreated verrucous form of CBM evidenced local immune response with high IL-17 expression and low expression of other cytokines, but this Treg/Th17 imbalance can provide proof of decreased immune response to the fungus.63
Siqueira et al. showed that the hyphae and muriform cells are capable of establishing murine CBM with skin lesions and similar histopathological features to those found in human tissue, and that the muriform cells are the most persistent fungal form, while the mice infected with conidia do not reach the chronic phase of the disease.64 They further demonstrated that in the damaged tissue, the presence of hyphae and especially of muriform cells, but not of conidia, correlates with the intense production of proinflammatory cytokines in vivo. The analysis of high throughput RNA sequencing showed strong regulation of genes related to fungal recognition, cell migration, inflammation, apoptosis, and phagocytosis in macrophages exposed in vitro to muriform cells, but not to conidia. They also demonstrated that only the muriform cells needed recognition of FcγR and dectin-1 for in vitro internalization and that this is the principal fungal form responsible for the intense inflammatory pattern observed in CBM, thereby elucidating the chronic inflammatory reaction seen in the majority of patients.64
Clinical ManifestationsCBM manifests clinically as oligosymptomatic or asymptomatic lesions, which would explain why patients only tend to seek medical care after months or even years of living with the disease. The initial lesion is usually on exposed areas, at the infection site, as a papule with centrifugal growth that evolves to any one of the several clinical forms. The polymorphism of CBM lesions encouraged some authors to develop various classifications of the clinical forms, most of which no longer used, while the classification proposed by Carrión in 1950 (Chart 1) is still in use.65
Clinical classification of chromoblastomycosis types according to Carrión (1950)
| Nodular | Fibrotic, erythematous-violaceous nodules, with smooth or hyperkeratotic surface |
| Verrucous or warty | Cauliflower-like, dry, hyperkeratotic lesions with black dots |
| Plaque (infiltrative or erythematous) | Erythematous or violaceous plaques, infiltrated, circumscribed, irregular, sharp and elevated edges, with black dots |
| Tumoral | Isolated or coalescent lobulated lesions, smooth or vegetative-like surface |
| Cicatricial or atrophic | Annular, serpiginous, or irregular lesions with centrifugal growth and central atrophic areas |
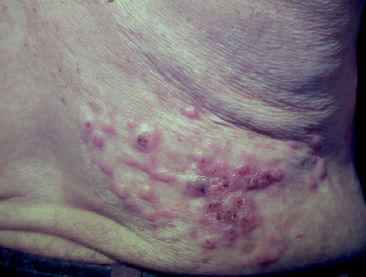
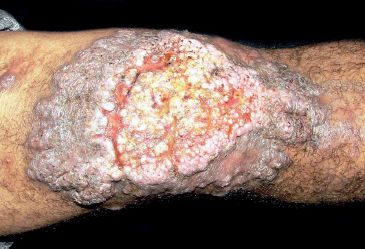
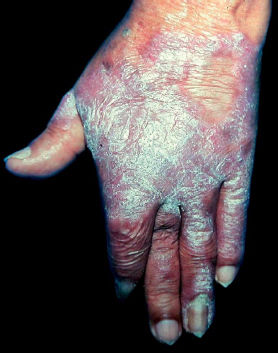
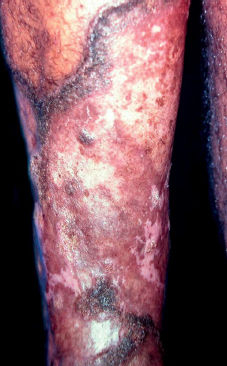
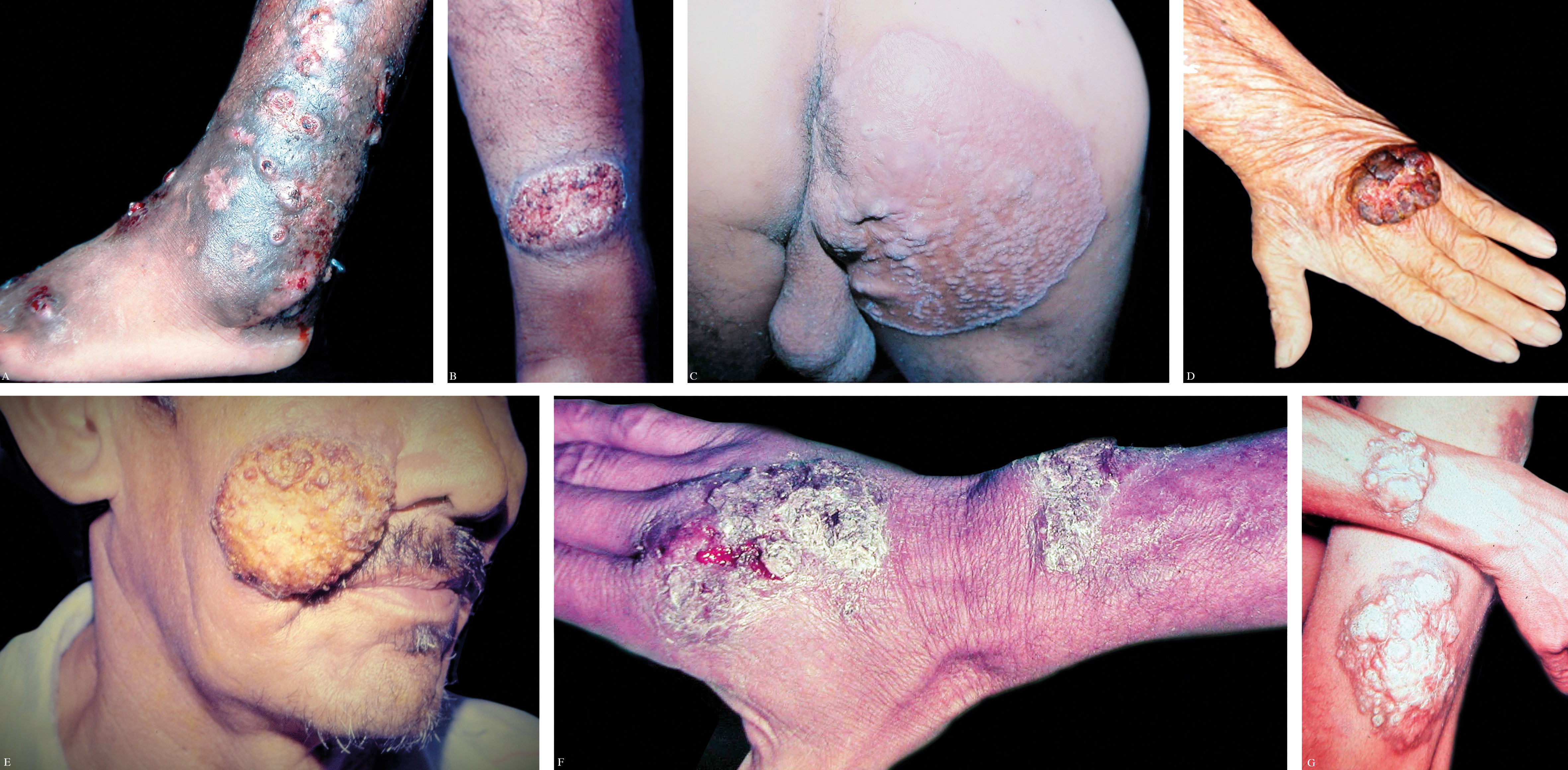
The initial lesion may remain circumscribed to the inoculation site for months or years, but it usually evolves to one of the lesion types characterizing the clinical polymorphism of CBM (Figure 1). By contiguity or lymphatic or hematogenous dissemination, metastatic lesions appear at other anatomic sites. In the nodular type, the clinical expression is that of fibrotic, erythematous-violaceous nodules with a smooth or hyperkeratotic surface (Figure 2). The verrucous type – with a higher prevalence, is characterized by lesions with a cauliflower appearance, dry, hyperkeratotic, with black dots, usually with abundant CBM agents, but ulceration occurs relatively frequently in this type of lesion (Figure 3). The plaque type displays erythematous or violaceous, infiltrated, circumscribed, irregular plaques, with sharp, elevated edges and black dots, in some cases with central scarring (Figure 4). The tumoral type is characterized by lobulated single or coalescent tumoral lesions with a smooth or crusted/scaly surface, or a vegetative appearance (Figure 5). In the cicatricial or atrophic type, the clinical appearance involves lesions with an annular, serpiginous, or irregular configuration and centrifugal growth with atrophic central areas, in some cases occupying large skin areas (Figure 6).
The great majority of CBM lesions are located on the lower limbs, especially in agricultural workers. Reports in the literature of different clinical features and other sites include: localized annular form, diffuse cutaneous form, on the scapular region, two cases on the axillae, on the abdomen, on the cornea, on the conjunctiva simulating melanoma, on the auricular region, and as a phagedenic ulcer on the face.43,66-76
Oral CBM was reported by Fatemi et al.77 Cases of extracutaneous CBM are rare, but hematogenous, lymphatic, or contiguous dissemination of the fungus has been known to metastasize to lymph nodes and lungs and produce osteolytic lesions underlying the skin lesion.78-80 There are reports of fatal cases of brain abscesses caused by F. monophora and F. pugnacius.22
Although most CBM patients are adults, cases of the mycosis have been reported in children and adolescents in endemic regions.27 The clinical manifestations of CBM display different degrees of severity, as follows:81
Mild form: single lesion, plaque or nodular type, less than 5 cm in diameter (Figure 7A).
Moderate form: single or multiple lesions, plaque, nodular, or verrucous (verruciform). When multiple, presence of one or various types of lesions located on one or two adjacent skin areas, less than 15cm in diameter (Figure 7B).
Severe form: any type of single or multiple lesion, adjacent or otherwise, covering extensive areas of the skin. When multiple, combined presence of one or several types of lesions (Figure 7C).
Patients report pruritis of variable intensity in the lesions, and pain in the presence of secondary infection. The following complications occur in CBM: bacterial infection, elephantiasis, and carcinomatous degeneration.82-90
Differential DiagnosisThe polymorphism of CBM lesions makes differential diagnosis mandatory with pathological processes of different etiologies, including: phaeohyphomycosis, paracoccidioidomycosis, sporotrichosis, lobomycosis (lacaziosis), coccidioidomycosis, North American blastomycosis, leishmaniasis, mycetoma, leprosy, cutaneous tuberculosis, non-TB mycobacterial infections, protothecosis, rhinosporidiosis, botryomycosis, tertiary syphilis, ecthyma, sarcoidosis, psoriasis, halogenoderma, and neoplasms, including squamous cell carcinoma, keratoacanthoma, and sarcoma (Figure 8).
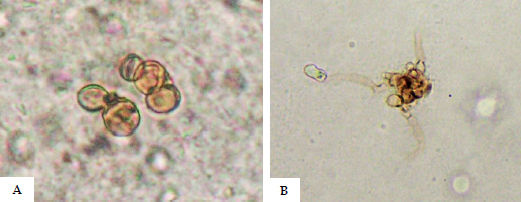
Laboratory DiagnosisDirect microscopy using potassium hydroxide (KOH) 10-20% or KOH/DMSO reveals muriform (sclerotic) bodies, pathognomonic of CBM regardless of the causative species (Figure 9A). Occasional dematiaceous hyphae may be associated with the muriform bodies in the material (Figure 9B). The specimens with the highest likelihood of a positive result are those from lesions with the so-called “black dots” that are visible on the lesion’s surface, representing transdermal elimination of the fungus. Miranda et al. (2005) used vinyl adhesive tape for the diagnosis of some deep mycoses, including CBM.91
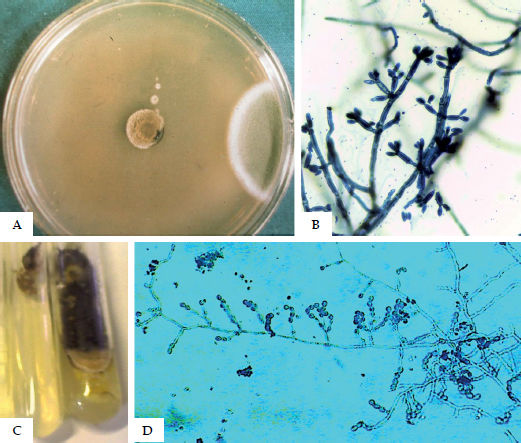
Fungal culture in Sabouraud agar is used to isolate and identify species, but the causative agents usually present very similar macromorphological characteristics. F. pedrosoi produces velvety, dark-brown, olive-green, or black colonies (Figures 10A and 10B). Phialophora verrucosa produces slow-growing, velvety, moss-green, brown, or black colonies. C. carrionii displays colonies very similar to those of F. pedrosoi (Figures 10C and 10D). R. aquaspersa colonies are velvety and moss-green to black.
Microculture yields three types of fruiting or sporulation: Cladosporium type – acrogenous catenulate sporulation, elliptical spores in chains; Phialophora type – conidiophore (phialide), flower vase-shaped with spores around the phialide; Rhinocladiella type – conidiophores formed along the hyphae and oval spores on the upper extremity (acrotheca) and along the conidiophore.
No intradermal tests for the disease have been standardized. Molecular biology techniques are currently essential to complete the diagnostic workup, and PCR tests have been developed to identify Fonsecaea species and C. carrionii.21,26,92 In light of the immune response in CBM patients, Oberto-Perdigon et al. used ELISA in 114 sera to assess the humoral response before, during, and after treatment employing a somatic antigen (AgSPP) of C. carrionii.93 The authors concluded that the method is valuable for diagnosis and assessment of therapeutic efficacy. However, PCR and ELISA are still not available in many endemic areas.
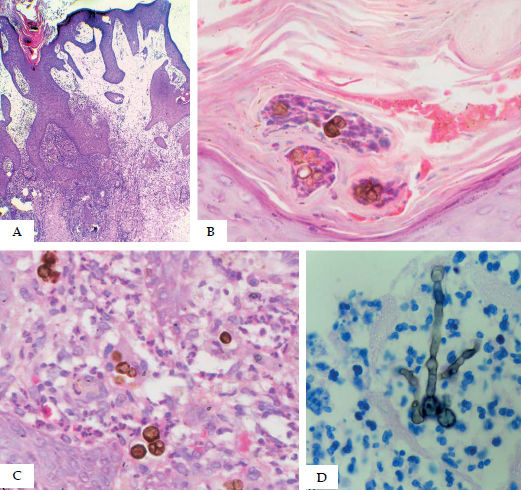
Histopathologically, CBM is characterized by an epidermis with hyperparakeratosis, pseudoepitheliomatous hyperplasia, intracorneal microabscesses, and transdermal elimination of fungi, the latter either inside or outside the microabscesses (Figure 11A and 11B). The dermis presents dense granulomatous inflammation with different degrees of fibrosis, consisting of mononuclear cells (histiocytes, lymphocytes, and plasma cells), epithelioid cells, giant cells (Langhans and foreign body types), and polymorphonuclear cells. Fungal cells with their characteristic micromorphology – round, dark-brown, thick-walled, 4-12 microns in diameter and with multiplanar reproduction, called muriform (sclerotic) bodies – are found in intraepidermal microabscesses in multinucleated Langhans and/or foreign body-type cells, in suppurative or tuberculoid granulomas, easily identified by hematoxylin-eosin staining (Figure 11C).
Histological features. A - Pseudoepitheliomatous hyperplasia, hyperparakeratosis, and dermis with edema and granulomatous inflammatory infiltrate (Hematoxylin & eosin, x40); B - Muriform cells in the stratum corneum with transdermal elimination(Hematoxylin & eosin, x400); C - Suppurated granuloma with muriform bodies inside giant cells (Hematoxylin & eosin, x400); D - Muriform cells and septated hyphae in abscess (Fite-Faraco staining) x100
Dimorphism may be observed, and it is possible to identify hyphae and muriform bodies in material from skin lesions.94 Pires et al., in a study of 65 patients that underwent histopathological examination with HE staining, found two main types of granulomatous tissue reaction: suppurative granuloma with abundant fungal cells, mostly from verrucous lesions, and tuberculoid granuloma, with few parasites, from plaque and atrophic lesions.95
There is an interesting report of detection of CBM agents using Ziehl-Neelsen and Wade-Fite staining, a useful approach in cases that are difficult with HE staining.96 Our study used Fite-Faraco staining and showed the dimorphism of the fungus – presence of muriform bodies associated with dematiaceous septated hyphae (Figure 11D). Saxena et al. (2015) detected abundant fungi under direct microscopy following intralesional infiltration of corticosteroids in a CBM lesion.97
TreatmentCBM is difficult to treat and associated with low cure rates and high relapse rates, especially in chronic and extensive cases. Treatment choice and results depend on the etiological agent, size and extent of the lesions, topography, and presence of complications.
Clinical cure can be defined as complete resolution of all the lesions, leaving scars. Mycological cure is proven by the absence of fungi on direct mycological examination and negative culture. Histopathology of the healed lesion shows atrophic epidermis and absence of granulomatous infiltrate and abscesses, which are replaced by cicatricial fibrosis associated with chronic inflammatory infiltrate and absence of fungi in serial slices.
Treatment consists of long periods of antifungal drugs, often combined with physical treatments like surgery, cryotherapy, and thermotherapy. Studies report highly variable clinical and mycological cure rates, ranging from 15% to 80%.98
Small and localized lesions can be removed surgically with wide margins, and antifungal agents are often used before surgery to downsize the lesion and later to avoid risk of relapse. Electrodissection and curettage are not recommended, since they can result in involvement of the lymphatic chain.98
Cryotherapy or cryosurgery with liquid nitrogen and thermotherapy (local heat to produce controlled temperatures of 42-45°C, which inhibit fungal growth) show minimal risk of adverse effects, and these treatment options are relatively inexpensive, but are more appropriate for single, limited lesions.99,100 Cryosurgery is relatively easy in technical terms, but the freezing time and depth have still not been standardized. Thermotherapy has been used less, and the cases with the best published results have been in Japan. The technique requires daily application of heat directly on the lesions for several hours, for 2-6 months.101,102
CO2 laser appears to be an interesting alternative for treatment of well-demarcated, localized CBM lesions. One advantage is the need for only a single treatment, which improves patient adherence. In addition, the cost of a single treatment is relatively low, with the advantage of no systemic toxicity.103 Combination treatment using CO2 laser and topical thermotherapy was used successfully in CBM by Hira et al.104
There was a recent promising description of photodynamic therapy in CBM.105,106 Hu et al. used oral terbinafine in combination with photodynamic therapy with 5-aminolevulinic acid in a case of CBM, with apparent clinical improvement in less than a year and no recurrence.107 Mohs micrographic surgery has been used to treat a variety of skin neoplasms with excellent results. The technique was used successfully to treat a localized cutaneous CMB lesion, with no recurrence of the lesion after a year of follow-up.108
The antifungals that have shown the greatest efficacy are itraconazole (200-400mg/day) and terbinafine (500-1000mg/day) for at least 6-12 months, preferably at higher doses if tolerated.109-113 Both drugs showed high in vitro activity against the causative agents of CBM.114,115 Pulse therapy with itraconazole was reported (400mg/day for 7 days/month) and proved more economical and effective and associated with better treatment adherence.116,117 In addition, the combination of an azole (itraconazole) and an allylamine (terbinafine) with different targets and synergistic effect has been used.118
Second-generation triazoles (voriconazole, ravuconazole, posaconazole, and isavocunazole) present in vitro activity against dematiaceous fungi and are promising drugs for treatment of deep dermatomycoses, but the experience is limited by the prohibitively high costs in their endemic configuration.106,119-122
Negroni et al.122 assessed six CBM patients that were resistant to conventional antifungal therapies and administered 800mg/day of posaconazole, with clinical success in five of the six patients. Posaconazole was well tolerated during long-term administration.122
Oral voriconazole was tested in some cases of treatment in resistant forms of CBM.106,120,121 Good clinical results were achieved with this drug, but adverse effects like visual disturbances and photosensitive skin reactions were observed.121
Among the other antifungals, ketoconazole is not recommended for prolonged treatment, because high doses are associated with toxicity. Fluconazole is also contraindicated, since in vitro studies have shown its limited activity against dematiaceous fungi.115
Fluorocytosine (converted into 5-fluorouracil in fungal cells) shows some degree of efficacy but is associated with high risk of development of resistance, besides being hepatotoxic and myelotoxic.123 With the emergence of more recent antifungals, the drug is now rarely used except in selected resistant cases.
Amphotericin B is ineffective as monotherapy, and even in combination with other antifungals the results are generally poor, but in vivo studies of a combination of amphotericin B and fluorocytosine have shown efficacy, indicating synergistic activity between the two.124
The combination of itraconazole and fluorocytosine has only been evaluated in a small number of patients but has proven very effective even in severe forms of subcutaneous mycoses.125 The pharmacological data showed an additive effect against fungi, where fluorocytosine causes suppression of the yeast’s DNA synthesis and itraconazole acts on the cell membrane, inhibiting the synthesis of ergosterol.126 Despite an insufficient number of cases for a detailed comparison, combination therapy with these two drugs can be an option in severe cases of CBM.126
The combination of antifungal drugs with immunoadjuvant compounds such as glucan and imiquimod have been investigated in recent years.5 Glucan, an injectable formulation of β1→3 polyglycoside obtained from Saccharomyces cerevisiae, is considered a modifier of the biological response due to its immunomodulatory potential, since it can be recognized by specific cell receptors and has the ability to enhance the host immune response, with the activation of macrophages, endothelial and dendritic cells, B and T-cells, and polymorphonuclear lymphocytes, with the resulting induction of expression of various cytokines like TNF-α, IL-6, IL-8, and IL-12.127 This treatment has been used successfully in some cases of leishmaniasis and paraccocidiodomycosis.128 In CBM, glucan was used in weekly subcutaneous infections combined with itraconazole, with a good clinical response.129,130 Azevedo et al. (2008) showed that after treatment with glucan, there was a significant increase in lymphoproliferation of the patient’s cells in the presence of F. pedrosoi antigens, with altered cytokine pattern, showing a decrease in the production of IL-10 and a significant increase in IFN-γ and TNF-α.129
Imiquimod is a synthetic compound with potent antitumoral, immunomodulatory, and antiviral action, which stimulates both the innate and acquired immune pathways.131 Souza et al. discovered an underlying defect in the innate recognition of CBM agents by toll-like receptors (TLRs), which can be restored by exogenous administration of a TLR agonist, including imiquimod.132 Imiquimod was used in a study with topical application 4 to 5 times a week in association with oral itraconazole, with a good clinical response.133
ConclusionsCBM is an important deep cutaneous mycosis which still causes major morbidity in affected patients. It is extremely difficult to treat, especially in the more severe clinical forms. Treatment generally consists of long periods of treatment with antifungals, often associated with physical treatments and immunotherapy. New studies are being published that help elucidate the immunopathogenesis of this mycosis, aimed at developing new therapies capable of modulating the host immune response.
Questions1. The following are causative species of CBM, except:
aFonsecaea pedrosoi
bCladophialophora carrionii
cRhinocladiella aquaspersa
dPenicillium marneffei
2. Melanin (dihydroxy naphthalene-melanin) in the wall of etiological agents of CBM is considered:
a A factor for resistance to antifungal agents
b A factor for virulence pathogenicity
c Only defines the color
d A reproductive factor
3. The following are considered clinical forms of CMB, except:
a nodular
b verrucous
c macular
d tumoral
4. As for antifungals used in the treatment of chromomycosis is CORRECT to afirm:
a Itraconazole and terbinafine show low in vitro activity against the etiological agents of CBM.
b Second-generation triazoles (voriconazole, ravuconazole, posaconazole) do not display in vitro activity against dematiaceous fungi.
c Ketoconazole is still recommended as an option for prolonged treatment.
d Fluconazole is not recommended, since in vitro studies showed little activity against dematiaceous fungi.
5. Concerning the use of amphotericin for the treatment of CBM, mark the CORRECT answer:
a Quite effective as monotherapy
b Safe drug for severe forms of the disease
c The combination with fluorocytosine has shown efficacy in in vitro studies
d The drug of choice for localized lesions
6. Cladosporium type fruiting is characterized by:
a Flower vase-shaped conidiophore (phialide) with spores around the phialide
b Conidiophores formed along the hyphae and oval spores on the upper extremity (acrotheca)
c Acrogenous catenulate sporulation, elliptical spores in chains.
d Brush-shaped conidiophore
7. As for the immunology of chromobastomycosis, mark the CORRECT answer:
a Verrucous lesions show a predominance of IL4 and IL10
b Humoral immune response predominates
c Th2 profile predominates in atrophic lesions
d Low expression of IL-17 in the lesions
8. The black dots in CMB lesions represent:
a Thrombosed vessels on the lesion’s surface
b Hematic crusts
c Transdermal elimination of the fungus
d Melanocyte proliferation
9. As for classification of CBM lesions according to Queiroz-Telles, the mild form is characterized by:
a Single lesion, plaque or nodular type, less than 5cm in diameter
b Single lesion, plaque or nodular type, less than 10cm in diameter.
c Single verrucous lesion less than 5cm in diameter.
d Multiple lesions, plaque or nodular type, less than 5cm in diameter.
10. The etiological agents of CBM belong to the family:
a Ajellomycetaceae
b Botryobasidiacea
c Herpotrichiellaceae
d Hydnaceae
Answers key
Tuberous sclerosis complex: review based on new diagnostic criteria. An Bras Dermatol. 2018;93(3):323-31.
1. A
2. D
3. B
4. D
5. D
6. A
7. D
8. B
9. C
10. C
Papers
Information for all members: The EMC-D questionnaire is now available at the homepage of the Brazilian Annals of Dermatology: www.anaisdedermatologia.org.br. The deadline for completing the questionnaire is 30 days from the date of online publication.