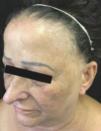
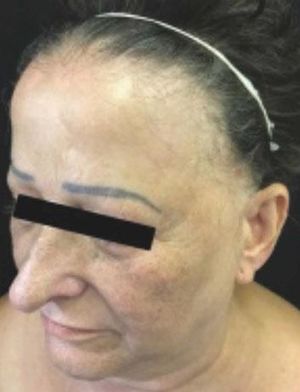
Background: Frontal fibrosing alopecia is a condition of unknown origin, histologically similar to classic lichen planopilaris and generally observed in postmenopausal women with alopecia of the frontal-temporal hairline.
Objectives: To describe the clinical, dermatoscopic, and histopathological characteristics and the treatment used in patients who have frontal fibrosing alopecia at the Alopecia Outpatient Clinic in a university hospital.
Methods: Retrospective descriptive study performed by reviewing medical charts and biopsies of the scalp.
Results: Sixteen patients were analyzed, all of them female, 93.75% of them postmenopausal, and 56.25% brown-skinned. All had frontal alopecia (100%), followed by temporal alopecia (87.5%) and madarosis (87.5%). On dermatoscopy, perifollicular erythema and tubular scales were found as a sign of disease activity. Of the patients, 68.75% had associated autoimmune diseases, including lupus, thyroid disease and vitiligo. Of the 13 biopsies from 8 patients, 10 showed microscopic aspects compatible with frontal fibrosing alopecia. Laboratory tests did not show major abnormalities and minoxidil was the most used treatment.
Study limitation: Data collection limited by the study’s retrospective design associated to flaws while filling in the medical charts and absence in standards to the collection and processing of the pathology and histopathological examination.
Conclusion: A demographical, clinical, and histopathological description of 16 patients diagnosed with frontal fibrosing alopecia, which remains a challenging disease, of unknown origin, and frequently associated with autoimmune diseases. This study reinforces literary findings. However, more research is needed to establish the pathogenesis and effective treatments.
It is estimated that the scalp has about 100,000 to 150,000 pilosebaceous follicles and hair rarefaction can generate psychological, social and cultural stigmas, and alopecia is a frequent dermatological complaint.
Signs of hair loss may be subtle and the dermatologist should perform a systematic approach to their evaluation, including detailed history, clinical, trichoscopic, laboratory and histopathological examination.
Alopecia can be classified into non-scarring and scarring. The scarring ones constitute a group of diseases that result in permanent loss of hair due to destruction of the hair follicles and their replacement by connective tissue.1 They can be subdivided into two categories: those in which the follicles appear to be the target of the inflammation (primary) and those in which the process destroys follicular structures in a non-specific way (secondary).2
Frontal fibrosing alopecia (FFA) is a type of primary cicatricial alopecia that affects predominantly postmenopausal women, affects the frontotemporal region, and can extend to the occipital region, being frequent the involvement of the eyelashes and also other sites of the body. Histopathologically, its findings are indistinguishable from those described in lichen planopilaris (LPP), including replacement of follicular units by connective tissue, perifollicular lymphocytic lichenoid infiltrate, basal layer vacuolar degeneration and perifollicular fibrosis, particularly around the infundibulum and isthmus.1
The aim of this study is to describe the demographic, clinical, dermatoscopic, and histopathological profile of patients with FFA as well as the treatments used. They were treated at the Alopecia Outpatient Clinic of a university hospital.
MethodsA retrospective cohort study was carried out based on the analysis of medical charts and review of slides containing histological sections stained by Hematoxylin & Eosin from patients who underwent a scalp biopsy for diagnostic confirmation. The patients studied were seen at the Hospital Universitário Clementino Fraga Filho (HUCFF), Universidade Federal do Rio de Janeiro (UFRJ), between January 2015 and December 2016.
All patients with clinical and/or histopathological diagnosis of FFA were included in the study. Those who did not present sufficient data due to lack of records were excluded.
The variables studied were: gender, age, color/race and menopausal status, comorbidities, associated symptoms (pruritus, pain, burning), area of alopecia, dermatoscopic findings (perifollicular erythema and tubular scales) and histopathological findings (hyperkeratosis, follicular ostium hypergranulosis, acanthosis, superficial perivascular mononuclear inflammatory infiltrate, pigmentary incontinence, vertical dermal fibrosis, perifollicular inflammatory infiltrate, perifollicular fibroplasia, vacuolar degeneration of the basal layer of the follicular epithelium and thinning of the external root sheath), laboratory tests (complete blood count, iron studies, liver function test, lipids, vitamin D, ESR, CRP, ANA, renal and hormonal functions) and treatment used.
The data were tabulated in spreadsheets and descriptive statistics were applied using the Excel program (Microsoft Office, version 2010).
ResultsA total of 171 patients were treated at the Alopecia Clinic between January 2015 and December 2016, of whom 112 (65.50%) had non-scarring alopecia, 51 (29.82%) had scarring alopecia and 8 (4.68%) patients with an association of both types.
Sixteen (9.36%) patients were diagnosed with FFA, all were female and the ages ranged from 29 to 80 years, with a mean of the age 62 (Figure 1). Most were postmenopausal (93.75%) and were brown-skinned (56.25%) (Table 1).
Demographic and clinical characteristics of patients with FFA
| Patient | Age | Skin color | Comorbidities | LPPig | Frontal Alopecia | Temporal Alopecia | Madarosis | Axillary and pubic alopecia | All-over--the-body alopecia | Alopecia on the limbs | Perifollicular erythema | Tubular scales |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | White | SAH, SLE, LED | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| 2 | 80 | White | None | No | Yes | Yes | Yes | No | No | No | Yes | Yes |
| 3 | 70 | White | SLE, hypothyroidism, Vitiligo | No | Yes | Yes | Yes | No | No | No | Yes | Yes |
| 4 | 55 | Brown | Vitiligo | Yes | Yes | Yes | Yes | Yes | No | No | No | No |
| 5 | 60 | Brown | SAH, DM, SLE, Hypothyroidism | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6 | 61 | Brown | None | No | Yes | No | Yes | No | No | No | Yes | Yes |
| 7 | 61 | Brown | SLE, Hashimoto’s thyroiditis, OLP | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| 8 | 29 | Brown | SLE, LED, vitiligo | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| 9 | 70 | Brown | SAH, DM, LED | No | Yes | Yes | Yes | No | No | No | Yes | No |
| 10 | 53 | White | Hashimoto’s thyroiditis | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| 11 | 65 | Brown | SAH | Yes | Yes | Yes | Yes | No | No | No | Yes | No |
| 12 | 73 | Black | SAH, LED, Hyperparathyroidism | No | Yes | No | No | No | No | No | Yes | No |
| 13 | 55 | Brown | None | Yes | Yes | Yes | Yes | No | No | No | No | Yes |
| 14 | 51 | White | SAH, LED, psoriasis | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 15 | 62 | Brown | SAH, hypothyroidism | Yes | Yes | Yes | No | No | No | No | Yes | Yes |
| 16 | 69 | White | HAS | No | Yes | Yes | Yes | No | No | Yes | Yes | No |
| Total (%) | 62 (average) | - | - | 5 (31,6%) | 16 (100%) | 14 (87,5%) | 14 (87,5%) | 6 (37,5%) | 2 (12,5%) | 5 (31,25%) | 14 (87,5%) | 10 (62,5%) |
SAH: systemic arterial hypertension; DM: diabetes mellitus; SLE: systemic lupus erythematosus; OLP: oral lichen planus. LED: discoid lupus erythematosus
Among the comorbidities investigated, 8 patients had discoid and/or systemic lupus erythematosus, 8 had hypertension, 6 had thyroid disease, 5 had lichen planus pigmentosus, 3 vitiligo, 2 patients were diabetic, 1 had psoriasis and 1 oral lichen planus (Table 1).
As for the site of alopecia, all had frontal alopecia and the majority temporal (87.5%) and madarosis (87.5%) (Table 1) (Figure 1). One patient was asymptomatic, 10 reported pruritus, 5 burning and 4 felt pain. Regarding dermatoscopy, 14 cases (87.5%) exhibited perifollicular erythema and 10 (62.5%) tubular scales (Table 1).
Among the laboratory results found in the charts, vitamin D was low in 3 out of 8 of them, reactive ANA in 3/7, elevated TSH in 3/9, elevated CRP in 5/6, elevated ESR in 4/9 patients. Blood count, iron studies, liver function test, lipids, and renal and hormonal functions showed no abnormalities.
Thirteen scalp biopsies were found from 8 patients. Four patients had one sample, three had two samples and one had three samples, one of the biopsies had been performed 8 years before the other two. Vertical serial cuts were performed in 12 samples and horizontal in one sample. Of the 12 biopsies whose vertical sections were analyzed, 9 biopsies presented hyperkeratosis, 9 showed follicular ostium hypergranulosis, 8 exhibited acanthosis and 5 had vertical dermal fibrosis. These findings were not possible to evaluate in the horizontally cut sample. Superficial perivascular mononuclear inflammatory infiltrate was seen in 13 cases and melanophages were found in 3 of them. Perifollicular mononuclear inflammatory infiltrate, in the infundibulum/isthmus area, was seen in 10 of the 13 cases and in 5 of them the inflammatory cells presented lichenoid distribution. In 7 biopsies there was perifollicular fibroplasia, in 4 vacuolar degeneration of the basal layer of the follicular epithelium was observed and in 4 thinning of the follicular epithelium was seen.
The treatment options used are described in table 2, with the majority of patients using 5% minoxidil, 0.1% tacrolimus, 0.05% clobetasol propionate and finasteride.
Treatments used by patients with FFA
| Patient | Minoxidil 5% | Clobetasol 5% | Prednisone | Tacrolimus 0.1% | Hydroxychloroquine | Finasteride | Doxycycline | Dapsone |
|---|---|---|---|---|---|---|---|---|
| 1 | X | X | X | X | X | |||
| 2 | X | X | X | |||||
| 3 | X | X | X | X | X | X | X | |
| 4 | X | X | ||||||
| 5 | X | X | X | |||||
| 6 | X | X | X | X | ||||
| 7 | X | X | ||||||
| 8 | X | X | X | X | ||||
| 9 | X | X | X | X | X | |||
| 10 | X | X | X | X | X | X | ||
| 11 | X | X | X | X | ||||
| 12 | X | X | X | |||||
| 13 | X | X | X | |||||
| 14 | X | X | X | X | X | |||
| 15 | X | X | ||||||
| 16 | X | X | ||||||
| Total (%) | 14 (87.5%) | 10 (62.5%) | 2 (12.5%) | 11 (68.75%) | 7 (43.75%) | 10 (62.5%) | 5 (31.25%) | 1 (6.25%) |
FFA was first described in 1994 by Steven Kossard.3 It is a primary lymphocytic scarring alopecia mainly characterized by progressive loss of frontotemporal hairline and eyebrows in postmenopausal women, although premenopausal women and men may also be affected. Vanó-Galvan et al studied 355 patients with FFA being 97% female and 86% postmenopausal, 98.5% Caucasians and with a mean age of 61 years; our results were similar to even though most patients were brown-skinned.4
In a study in 2014, with 62 patients with FFA, frontotemporal alopecia was observed in all patients, then the loss of eyebrows (81%) axillary and pubic hair (53%) and in the rest of the body (19%).5 Similar data have been found in our study and in other publications.6-9 It is noteworthy that eyebrow loss may be an early presentation and it is recommended that the dermatologist biopsy the site to diagnose FFA early and avoid permanent hair loss.10
AFF may have an asymptomatic course, but most patients report burning, itching or pain in the scalp.5,7 In this study, pruritus was the most reported complaint.
Trichoscopy is an easily accessible, non-invasive and economical tool, being important for the assessment of the severity and for differential diagnoses with other types of alopecia. Signs of local inflammation of hair follicles such as tubular scales at the base of the stems and perifollicular erythema are indications of disease activity and indicate the need for treatment.1,5 The absence of follicular ostia is seen in the final phase of the disease. According to Fernández-Crehuet et al, in a review of 249 cases, the presence of tubular scales at the base of the hair was seen in 89% of the cases, perifollicular erythema in 77% and isolated hairs in 67.9%; our cases showed similar data.11
The pathogenesis of FFA is not yet understood. It is suggested to be an immunomediated disease with the participation of cytotoxic T cells in the inflammatory process. The destruction of the follicle would be due to a continuous inflammatory response triggered by proinflammatory cytokines.
Since FFA is frequently concomitant with other autoimmune diseases such as thyroid disease, vitiligo, lupus, Sjogren’s Syndrome and psoriasis, it is possible to assume common pathogenic mechanisms for these conditions.5,8,9,12,13 This association between FFA and autoimmune diseases was found in 30% of patients by Mac Donald et al,8 and, in our study it was observed in 68.75% of the cases. Hypothyroidism was reported more frequently in patients with FFA than the general population (15% to 4.2%).4 In addition, autoimmune diseases could guide early detection in those patients with a genetic predisposition to the development of FFA, as well as the diagnosis of FFA should guide the investigation of other autoimmune diseases, especially thyroid disease and lupus. Other hypotheses have also been cited by some authors, such as heredity and hormonal factors; however, further studies are needed to define the pathogenesis. 9,14,15
Lichen planus pigmentosus is a macular variant of lichen planus characterized by brown, hyperchromic and reticulate patches in photoexposed areas and flexures. In 2013, Dlova reported the concomitance of LPPig with FFA in 54.54% of the patients studied, most of them in pre-menopause and with high phototype; in our study, it was found in 31.6% of the cases. 16
There are few reports in the literature of laboratory abnormalities; however, Vañó-Galván et al recommend investigating the presence of associated thyroid disease.4,7,9 In our series, laboratory data were not available for all the patients, due to the fact that this is a retrospective study. Despite that, positive ANA, increased CRP, ESR and TSH; and low vitamin D were found.
The histopathological picture of FFA was described by Kossard in 1994 after analysis of scalp biopsies of 6 postmenopausal patients.3 Samples were obtained from the frontal hairline and showed a lymphocytic inflammatory infiltrate concentrated around the follicular isthmus/infundibulum and perifollicular fibrosis, which are indistinguishable from those observed in lichen planus pilaris. These aspects were confirmed by subsequent reports and are in accordance with the data observed in the present study, in which 10 of the 13 samples analyzed presented perifollicular inflammatory infiltrate in the isthmus/infundibulum area and 7 of them had associated perifollicular fibrosis. 17,18
There is no standardized therapy for FFA. The treatment aims to reduce inflammation, decrease sequelae and slow the progression of the disease. Rácz et al carried out a literature review of the treatments used in 114 patients and observed that 5-alpha reductase inhibitors were used most of the time, resulting in a good clinical response in 45% of them. However, it is questioned whether its effectiveness could be due to the improvement of associated androgenetic alopecia.19 The same questioning is required for the use of minoxidil, since it increases capillary density in the treated area, but does not seem to modify the course of the disease. Other options include hydroxychloroquine/chloroquine, cyclosporine, doxycycline/minocycline, mycophenolate mofetil, topical and oral corticosteroids, and calcineurin inhibitors.1,4,5,9,19 Rakowska et al have recently shown good results with retinoids, isotretinoin and acitretin.20 Minoxidil was therapy used in our study. The use of immunobiologics may be an alternative in the future and there is a report of complete resolution of LPP in a patient with chronic juvenile arthritis treated with rituximab (anti-CD20 antibody).21 Hormone replacement therapy shows no efficacy.3 Fertig and Tosti suggested that oral finasteride associated with hydroxychloroquine, topical inhibitors of calcineurin (tacrolimus) and Excimer Laser would have positive results in the treatment of FFA.22 However, controlled and randomized clinical trials are still required.
ConclusionFFA remains a challenging disease, of unknown origin, histologically similar to classic LPP and is commonly seen in postmenopausal women. Although clinically characterized as frontotemporal hairline alopecia, madarosis and perifollicular erythema, there is no established effective therapy. We present the demographic, clinical and histopathological description of 16 patients diagnosed with FFA, mostly treated with minoxidil. It should be noted that most of them had concomitant autoimmune disease, such a finding could possibly serve as an indicator for investigation in the setting of AFF. Randomized and controlled multicenter trials are needed to attempt to establish the pathogenesis and an appropriate treatment.