Paraneoplastic pemphigus is a rare and severe autoimmune blistering disease characterized by mucocutaneous lesions associated with benign and malignant neoplasms. Diagnostic criteria include the presence of chronic mucositis and polymorphic cutaneous lesions with occult or confirmed neoplasia; histopathological analysis exhibiting intraepidermal acantholysis, necrotic keratinocytes, and vacuolar interface dermatitis; direct immunofluorescence with intercellular deposits (IgG and C3) and at the basement membrane zone (IgG); indirect immunofluorescence with intercellular deposition of IgG (substrates: monkey esophagus and simple, columnar, and transitional epithelium); and, autoreactivity to desmogleins 1 and 3, desmocollins 1, 2, and 3, desmoplakins I and II, envoplakin, periplakin, epiplakin, plectin, BP230, and a-2-macroglobulin-like protein 1. Neoplasias frequently related to paraneoplastic pemphigus include chronic lymphocytic leukemia, non-Hodgkin lymphoma, carcinomas, Castleman disease, thymoma, and others. Currently, there is no standardized treatment for paraneoplastic pemphigus. Systemic corticosteroids, azathioprine, mycophenolate mofetil, cyclosporine, rituximab, cyclophosphamide, plasmapheresis, and intravenous immunoglobulin have been used, with variable outcomes. Reported survival rates in 1, 2, and 5 years are 49%, 41%, and 38%, respectively.
Paraneoplastic pemphigus (PNP) is a rare autoimmune blistering disease characterized by polymorphous cutaneous lesions and chronic and recalcitrant mucositis, first reported by Anhalt et al.1 PNP is associated with benign and malignant hematologic neoplasms and solid tumors.1
In 1990, Anhalt et al. described PNP, a novel variant of pemphigus with distinct clinical and laboratory findings. They suggested five diagnostic criteria: 1) painful mucosal erosions and a polymorphous cutaneous eruption, with an occult or confirmed neoplasm; 2) histologic findings – intraepidermal acantholysis, keratinocyte necrosis, and vacuolar interface dermatitis; 3) direct immunofluorescence (DIF) with intercellular deposition of IgG and C3 in the epidermis and granular deposition of C3 along the epidermal basement membrane zone (BMZ); 4) indirect immunofluorescence (IIF) with intercellular IgG deposition (monkey esophagus substrate), resembling the IIF findings of pemphigus vulgaris (PV) or pemphigus foliaceus (PF); positive IIF with simple, columnar, and transitional epithelia (not found in PV and PF); and 5) immunoprecipitation reactivity for four proteins (250 kDa, 230 kDa, 210 kDa, and 190 kDa).1
Further studies reported the role of potential antigenic targets in the pathogenesis of PNP: envoplakin, periplakin, desmoplakin I, desmoplakin II, epiplakin, plectin, BP230, desmoglein 1 (Dsg1), desmoglein 3 (Dsg3), desmocollin 1 (Dsc1), desmocollin 2 (Dsc2), desmocollin 3 (Dsc3), and a-2-macroglobulin-like protein 1 (A2ML1).2-9
In 2001, Nguyen et al. proposed the term “paraneoplastic autoimmune multiorgan syndrome” (PAMS) replacing PNP, to emphasize the multi-systemic aspects of the disease.10 The terms PNP and PAMS have been discussed in the literature; however, due to the pemphigus resemblance of the pathogenic antibody-mediated role of the disease, the term PNP is preferred.10-15
PNP is a rare condition, with around 500 cases described in the literature. The onset of PNP usually occurs between 45 and 70 years of age, with no gender differences.16,17 There are also reports of PNP in children and adolescents.18
PathogenesisThe entire pathogenesis of PNP remains to be defined, but humoral and cell-mediated immune responses do play a relevant role.
Antibody-mediated immunityThe pathogenic IgG autoantibodies in PNP are polyclonal.1 PNP patients show IgG autoantibodies directed against multiple antigens: Dsg3 and/or Dsg1; Dsc1, Dsc2, and Dsc3; proteins of the plakin family (envoplakin, periplakin, desmoplakin I, desmoplakin II, epiplakin, plectin, and BP230), in addition to a protease inhibitor, A2ML1.1,4-6,15,16,19,20
The reported autoantibody profile is heterogeneous: one study found that antibodies against Dsg3 and Dsg1 are present in 100% and 64% of PNP patients, respectively; the role of these antibodies has been demonstrated and is linked to the induction of blister formation.21 Another report utilizing ELISA with 79 PNP patients revealed IgG anti-Dsc1, anti-Dsc2, and anti-Dsc3 reactivity of 16.5%, 36.7%, and 59.5%, respectively; the role of these antibodies is still unknown.22
Plakins are molecules located at the intracellular plaques of the desmosomes and hemidesmosomes.23 The most common antibodies against proteins of the plakin family in PNP are anti-envoplakin and anti-periplakin antibodies.24 IgG autoantibodies against cytosolic proteins of the plakin family do not attack directly in vivo, an with unclear role in the pathogenesis of PNP.21
The possible antibody-mediated mechanisms in PNP related to neoplasias are as follows:16,25-28
- 1.
Tumor-induced production of autoantibodies against epithelial proteins;
- 2.
Cross-reactivity of tumor and epithelial antigens;
- 3.
Elevated IL-6 leading to B-cell differentiation and immunoglobulin production;
- 4.
Epitope spreading (interface dermatitis induced by the neoplasia exposes epidermal epitopes with autoantibody production against multiple epidermal proteins).
Cytotoxicity, as a cell-mediated mechanism, has a key role in the lichenoid mucocutaneous findings in PNP; however, this role has not been fully understood.10,29
Variable genetic susceptibility polymorphisms among different races have been observed in PNP. The presence of HLA-DRB*03 was significantly associated with PNP in white French individuals (p = 0.03) in comparison to patients with PF, PV, and healthy individuals; in counterpart, the presence of HLA-Cw*14 was increased in Han Chinese patients with PNP.30,31
Clinical FeaturesPatients with PNP usually present severe systemic findings of malaise, weakness, and weight loss (consumptive syndrome due to underlying disease or decreased intake associated to painful oral lesions). As for skin and mucosal lesions, PNP shows polymorphous features. A clinical report of 88 PNP patients revealed that 82/88 (93%) showed oral involvement and 59/88 (67%) had mucocutaneous lesions.32 Mimouni et al. evaluated 14 children and adolescents with PNP and found diffuse oral erosions in 14/14 (100%), lichenoid lesions in 8/14 (57%), other cutaneous findings in 3/14 (21%), genital erosions in 7/14 (50%) and ocular involvement in 6/14 (43%).18
PNP lesions may clinically resemble pemphigus vulgaris, bullous pemphigoid, erythema multiforme, lichen planus, and graft-versus-host disease.1,10,24
The variable clinical presentations of PNP may be related to the predominant pathogenic mechanisms involved: antibody-mediated immunity - PNP lesions resembling pemphigus vulgaris and bullous pemphigoid; cell-mediated cytotoxicity - PNP lesions resembling erythema multiforme, graft-versus-host disease, and lichen planus.
The distinct clinical presentations in PNP may coexist or may evolve during follow-up.
Mucosal lesions are usually the first manifestations of PNP, preceding skin lesions for days, weeks, or months. A study of PNP patients from Japan, Korea, the United States, and Europe revealed the striking presence of mucosal lesions: oral in 82/88 (93%), ocular in 33/81 (41%), nasal in 9/77 (12%), and genital in 28/79 (27%); 24/88 (27%) presented only mucosal lesions.32
Mucosal lesions occur as erosions and crusting in the mouth, nose, pharynx, larynx, esophagus, eyes, and anogenital area.
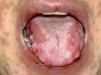
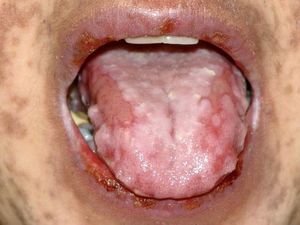
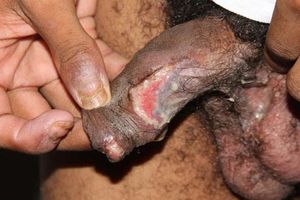
Oral lesionsOral lesions are one of the most remarkable manifestations in PNP, with extensive and recalcitrant erosive mucositis.10,32,33 They are chronic, painful erosions and crusting, and usually affect the vermilion of the lips, also covering the skin around the mouth. The lateral border of the tongue is a frequent site of involvement and erosions may occur throughout the entire buccal mucosa (Figure 1). Vesicles and blisters are seldom observed.20 Oral lesions may be the unique expression of PNP.
Cutaneous lesionsCutaneous lesions are polymorphous and may occur with variable presentations. Ohzono et al. found only 32 out of 88 PNP patients with characteristic skin lesions, distributed as follows: 18/32 (56%) with erythema multiforme-like lesions, 9/32 (28%) with pemphigus vulgaris-like lesions, 4/32 (13%) with lichen planus-like lesions, and 1/32 (3%) with bullous pemphigoid-like lesions.32
- -
Cutaneous lesions are characterized as follows:10,15,34
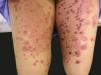
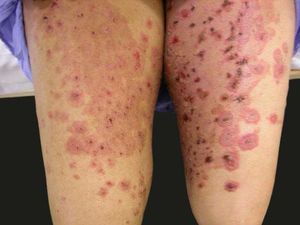
Erythema multiforme-like lesions: targetoid erythematous papules, with central blisters in the trunk and extremities, resembling erythema multiforme or toxic epidermal necrolysis in more advanced cases (Figure 2);
- -
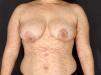
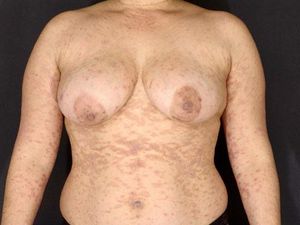
Pemphigus vulgaris-like lesions: confluent erythema in the V area and dorsum; flaccid blisters with extensive erosions and epidermal detachment areas (Figure 3);
- -
Lichen planus-like lesions: isolated or generalized lichenoid papules in the trunk, neck, and extremities (Figure 4);
- -
Bullous pemphigoid-like lesions: scaly erythematous papules with sero-hemorrhagic tense blisters;
- -
Graft-versus-host disease: generalized scaly red-brown papules.
Vesicles and blisters in PNP may often occur over areas of erythema, seldom over normal, non-inflammatory skin.34 Pustular lesions have been reported in PNP;35 however, the scalp is usually not involved.15 Lesions on palms and soles with sparing of the scalp are clinical features that differentiate PNP from PV.
Ocular lesionsOcular involvement is demonstrated in 41%–70% of PNP patients.32,36 The main ocular findings are bilateral conjunctival hyperemia and erosions, pseudomembranous conjunctivitis, bilateral corneal erosions, early symblepharon formation, forniceal shortening, and thickening of the palpebral margin.34,36,37 Burning and pain, mucus discharge, and decreased visual acuity are the most frequent ocular symptoms and signals.36,37
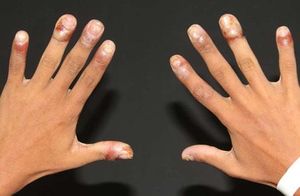
Involvement of other mucosal surfaces and organsNose and genital lesions were reported, with erosions (12%) and crusting (35%) detected in PNP patients (Figure 5).32 Other mucosal surfaces and organs – such as the pharynx, larynx, esophagus, and anus – are also affected. Pulmonary involvement occurs as obstructive lung disease and may lead to obliterative bronchiolitis and death. Pulmonary disease in PNP may range from 59.1% to 92.8%.16,38
Clinical and Laboratory DiagnosisIn patients with chronic, recalcitrant mucosal erosions that may also develop polymorphic cutaneous lesions in the presence of an underlying neoplasm, the diagnosis of PNP should be considered. Mucous involvement often precedes the development of cutaneous lesions that may resemble lichen planus, erythema multiforme, toxic epidermal necrolysis, or even be indistinguishable from other autoimmune blistering disorders such as PV, bullous pemphigoid, and mucous membrane pemphigoid.1,39,40
Neoplasia assessmentApproximately one-third of the PNP patients have a previously confirmed neoplasia, whereas the majority still requires a careful investigation to establish the diagnosis of an occult tumor.41 Hematologic malignancies are the most frequently associated neoplasias, mainly represented by non-Hodgkin lymphoma (38.6%–45%), chronic lymphocytic leukemia (18.4%), Castleman disease (15%-18.4%), thymomas (5.5%-7%), Waldenström macroglobulinemia (1.2%), Hodgkin’s lymphoma (0.6%), and monoclonal gammopathy (0.6%). PNP in children and adolescents is often associated with Castleman disease.18 Solid tumors have also been described in 14.8%-17% of the patients with PNP, among which 8.6% have epithelial origin (carcinoma of the breast, colon, pancreas, prostate, and skin) followed by 6.2% mesenchymal-derived neoplasms (different types of sarcomas).32,40 The laboratory and imaging evaluations recommended for screening of neoplasias are summarized in chart 1.42
Screening for underlying neoplasia in paraneoplastic pemphigus42
| Laboratory testing | Imaging exams |
|---|---|
| Complete blood count | Computed tomography: chest, abdomen, pelvis |
| Lactate dehydrogenase | Endoscopy |
| Colonoscopy | |
| Protein electrophoresis | |
| Mammogram |
Persistent oral erosions involving the lateral border of the tongue and extending to the vermillion of the lips are the most common manifestations of PNP; they represent the exclusive clinical presentation in 27% of patients.1,32,40,43
Lesions may also affect the nasopharynx, oropharynx, and larynx, leading to odynophagia, dysphagia, and hoarseness that require assessment by an ear, nose, and throat specialist. Patients often present undernourishment due to decreased protein and caloric intake caused by painful oral mucosal lesions and may require a nasoenteric tube or gastrostomy and nutritional support by a nutrologist.32,44-46
Ocular lesions affect 70% of the patients that present with scarring keratoconjunctivitis, with erosions and symblepharon resembling mucous membrane pemphigoid.34,36 Early ophthalmological evaluation is crucial to prevent irreversible loss of visual acuity.36
Progressive dyspnea and hypoxemia are the most frequent and warning signs of bronchiolitis obliterans and should prompt an immediate examination by a pulmonologist. Bronchiolitis obliterans occurs in 30% of the patients with PNP and is more commonly associated with Castleman disease in children.43,47 It manifests as an obstructive and/or restrictive pulmonary function test with bronchiectasis on chest computed tomography.15,48 Deposition of IgG in the bronchial epithelium has been demonstrated in autopsy specimens and induces detachment of dyskeratotic epithelial cells that migrate into smaller airways.10 Subsequent inflammation mediated by CD68+ cells and lymphocytes promotes irreversible fibrosis and obstruction.15,49
Immunopathological analysisThere is a close correlation between the clinical aspects of the mucocutaneous lesions with the histopathological findings in PNP.1
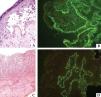
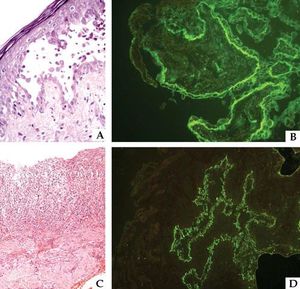
In patients with a predominantly cytotoxic response represented by lichenoid or erythema multiforme-like lesions, histopathological evaluation often demonstrates acanthosis, dyskeratotic and necrotic keratinocytes, vacuolar degeneration, and lymphocyte exocytosis.50 Keratinocyte necrosis may even extend to all epidermal layers.50,51 An intense inflammatory infiltrate with lymphocytes, mononuclear cells, and natural killer cells is an additional finding (Figure 6).
Immunopathological findings in paraneoplastic pemphigus: histopathological analysis showing (A) suprabasal acantholysis with acantholytic and apoptotic cells (Hematoxylin & eosin, x40) and (C) lichenoid interface dermatitis (Hematoxylin & eosin, x20). (B) Direct immunofluorescence with IgG deposition between epidermal keratinocytes and along the basement membrane zone; (D) indirect immunofluorescence studies in vesical murine epithelium with IgG deposits
However, blisters and erosions are commonly seen in individuals with a mainly humoral response that is histologically characterized by epidermal acantholysis with suprabasal or subepidermal detachment (Figure 6).
Presence of polymorphic lesions may be accompanied with mixed histopathological findings combining interface dermatitis with acantholytic cells.50
One of the key diagnostic hallmarks is the demonstration of the presence of autoantibodies directed against antigens of the plakin family using immunofluorescence studies, enzyme-linked immunosorbent assay (ELISA), and immunoblotting/immunoprecipitation.
DIF on perilesional skin may display IgG and/or C3 deposition on the keratinocyte cell surface and along the BMZ, with a sensitivity of 41% and specificity of 87% (Figure 6).50,51
IIF utilizing monkey esophagus or normal human skin as substrate usually displays intercellular deposition of IgG.50 Immune complex binding may also occur in gastrointestinal, respiratory, genitourinary, myocardium and thyroid epithelium, thus reinforcing the multi-systemic involvement in PNP.1,50 Even though envoplakin, periplakin, and desmoglein are not expressed in transitional epithelium, murine bladder contains more desmoplakin than the human skin.1,52 Therefore IIF with mouse or rat bladder as a substrate has a higher sensitivity (86%) and specificity (98.9%) for the diagnosis of PNP (Figure 6).43,53,54 Previous studies have demonstrated that anti-desmoplakin antibody positivity by IIF is not specific to PNP and may also occur in patients with PV and PF, thereby suggesting that additional studies including ELISA, immunoprecipitation, and immunoblotting may be required to confirm PNP diagnosis.55,56
Quantitative analysis of autoantibodies may be performed using ELISA assembled with recombinant fragments of envoplakin, periplakin, Dsc1-3, Dsg1, Dsg3, BP180, and BP230.
Immunoblotting and immunoprecipitation are the gold standard methods for the diagnosis of PNP to reveal circulating autoantibodies against desmosomal and hemidesmosomal antigens (Chart 2).8,22,32,39,57-59 Nevertheless, both techniques are not widely available, especially outside academic research centers.
Main antigenic targets in paraneoplastic pemphigus8,22,32,39,57,58
| Antigen | Molecular weight | Location | Diagnostic relevance |
|---|---|---|---|
| Plectin | > 400 kDa | Hemidesmosome | Plakin family58 |
| Epiplakin | > 400 kDa | Plakin family58 | |
| Desmoplakin I | 250 kDa | Desmosome | Plakin family58 |
| Bullous pemphigoid antigen 1 | 230 kDa | Hemidesmosome | Plakin family58 |
| Desmoplakin II | 210 kDa | Desmosome | Plakin family |
| Envoplakin | 210 kDa | Desmosome | Plakin family; higher diagnostic sensitivity59 |
| Periplakin | 190 kDa | Desmosome | Plakin family; higher diagnostic sensitivity59 |
| α-2-macroglobulin-like protein 1 (A2ML1) | 170 kDa | α-2-macroglobulin 1, protease inhibitor expressed in upper epidermal layers | Higher diagnostic sensitivity;59 early PNP onset and lack of ocular involvement8 |
| Desmoglein 1 | 160 kDa | Desmosome | |
| Desmoglein 3 | 130 kDa | Desmosome | Association with bronchiolitis obliterans and genital lesions32 |
| Desmocollin 1-3 | 80-100 kDa | Desmosome | Anti-Dsc2 and Anti-Dsc3 negativity correlate with lower ocular involvement32 |
There is currently no consensus for the diagnosis of PNP. Anhalt et al. initially described five main diagnostic features (Chart 3).1,8,15,32,42,51,59,60 However, the demonstration of new antigenic targets and the development of other techniques to identify the presence of autoantibodies reinforces the need to update the diagnostic criteria.22,61 Joly et al. determined that the occurrence of lymphoproliferative disease, positive IIF with rat bladder, and presence of anti-periplakin and anti-envoplakin with immunoprecipitation or immunoblotting are the most sensitive and specific findings for the diagnosis of PNP.51
Diagnostic criteria for paraneoplastic pemphigus (Anhalt et al., 1990)1
| Features | Criteria | Limitations |
|---|---|---|
| Clinical | Painful mucosal involvement with polymorphic skin lesions with an underlying neoplasm | Exclusive oral mucosal lesions have been described,32 as well as cases without a concomitant neoplasia32,61 |
| Histopathological | Acantholytic and necrotic keratinocytes, and vacuolar degeneration | Subepidermal detachment may be present15,42 |
| Immunofluorescence | IgG and C3 deposition in the intercellular spaces as well as along the basement membrane zone | Sensitivity and specificity for direct immunofluorescence are 41% and 87%, and for indirect immunofluorescence, 86% and 98%, respectively51 |
| Circulating autoantibodies binding to the epidermal cell surface of cutaneous and mucosal samples, as well as to columnar and transitional epithelia | Maximum sensitivity and specificity may be obtained with the combination of indirect immunofluorescence (rat bladder) with immunoblotting59 | |
| Immunoprecipitation | Positivity to proteins of 250, 230, 210 and 190 kDa | Additional antigenic targets have been demonstrated8,32 |
Due to the polymorphic clinical presentation of PNP, a broad list of differential diagnoses has to be considered, which are summarized in chart 4.
Differential diagnoses of paraneoplastic pemphigus39
| Autoimmune diseases | Inflammatory disorders |
|---|---|
| Pemphigus vulgaris | Erythema multiforme |
| Mucous membrane pemphigoid | Lichen planus |
| Bullous pemphigoid | Stevens-Johnson syndrome |
| Lichen planus pemphigoid | Toxic epidermal necrolysis |
| Epidermolysis bullosa acquisita | Graft-versus-host disease |
PNP is a life-threatening disease with variable outcome. Initial reports of a high one-year mortality rate of up to 90% rely on single cases or small series including patients with severe disease, possibly leading to a publication bias.46 In recent years, studies including larger series of cases with a broader spectrum of clinical presentations have described patients with better outcomes; the authors demonstrated survival rates at one, two, and fives years of 49%, 41%, and 38%, respectively.62
A retrospective, multicentric study of 53 consecutive cases of PNP during an 18-year period included patients with both moderate and severe presentations. Diagnosis was confirmed according to the criteria established by Anhalt et al. at a mean age of 59 years.1 The most frequently associated neoplasias were chronic lymphocytic leukemia (30.2%), followed by non-Hodgkin lymphoma (26.4%), carcinoma (18.9%), Castleman disease (9.4%), and thymoma (7.5%). The authors identified, after an age- and sex-adjusted multivariate analysis, that the presence of erythema multiforme-like lesions was the main criterion of decreased survival (p = 0.05), especially in patients with severe mucocutaneous lesions. Non-Hodgkin lymphoma was correlated to poorer outcome, whereas Castleman disease and thymoma were correlated to a better prognosis, possibly attributable to a younger age of presentation and lack of treatment with chemotherapy.62
Several factors may influence the prognosis of this paraneoplastic syndrome, including the nature of the underlying neoplasia, the severity of mucous involvement, and the response to treatment and its associated complications.32,39,62 Successful removal of solid tumors or remission of hematologic neoplasia with chemotherapy is crucial for the improvement of PNP, as autoantibodies produced by tumoral cells are implicated in the pathogenesis of mucocutaneous lesions.63,64 Total resection of thymoma and Castleman’s tumor enables a progressive reduction of circulating autoantibodies within six to eight weeks that correlates with the improvement of cutaneous lesions.25,38 However, incomplete removal or recurrence of the neoplasia is related to relapse of PNP and death.25
Recalcitrant, painful oral mucous lesions are one of the main features of the disease and may limit dietary intake, leading to malnutrition and weight loss that further compromise the patient’s clinical conditions.45 Immunosuppressive medications utilized as an attempt to improve mucocutaneous involvement may increase the risk of infectious complications and lead to sepsis, which is the second most frequent cause of death in PNP.32
Myasthenia gravis is a potential complication observed in 35% of the patients with PNP, mainly associated with thymoma.65 It manifests as muscle weakness, fatigue, and dyspnea.11,65 This autoimmunity against acetylcholine, titin, and ryanodine receptors may be triggered by intratumoral auto-reactive CD8+ T cells.42 A retrospective study evaluated the presence of serum autoantibodies associated to myasthenia gravis in 58 PNP patients with myasthenia gravis, and compared them to 69 PNP patients without myasthenia gravis, 20 PV patients, 20 PF patients, 21 patients with connective tissue disease, and 49 healthy individuals. Both anti-acetylcholine receptor and anti-acetylcholinesterase antibodies were significantly increased in PNP patients with myasthenia gravis in comparison to controls, and also correlated with dyspnea (p < 0.05). Increased levels of anti-titin and anti-ryanodine receptor antibodies occurred in PNP patients with muscle weakness (p < 0.05), similarly to previous studies that demonstrated a positive correlation between the levels of both antibodies with the severity of myasthenia gravis.65-67
Bronchiolitis obliterans is a severe manifestation of PNP and one of the leading causes of mortality. Desmoplakins I and II, envoplakin, periplakin, and BP230 are expressed in the bronchial epithelium.10,46 Autoantibodies against these intercellular and intracellular adhesion glycoproteins trigger bronchial epithelial desquamation and sloughing into the airway lumen, resulting in epithelial inflammation and subsequent fibrosis.49 Irreversible terminal airflow obstruction may lead to end-stage respiratory failure and death.15
Ohzono et al. demonstrated a correlation between circulating anti-Dsg3 and development of bronchiolitis obliterans in a retrospective study including 104 patients with PNP.32 In this study, the autoantibody response against epiplakin, a protein strongly expressed in human and mouse bronchiole epithelium, was also evaluated. The presence of anti-epiplakin antibodies significantly correlated with development of bronchiolitis obliterans (p = 0.03) and increased mortality (p = 0.03) in Japanese patients with PNP, but this correlation was not observed in the European population.68 Anti-epiplakin antibodies injected into mice induce a disruption of bronchial epithelium integrity and mononuclear cell inflammation, providing evidence of its pathogenic role in bronchiolitis obliterans.68
TreatmentThere is currently no standard treatment for PNP due to lack of randomized controlled trials, given the rarity and severity of the disease, and the multitude of clinical presentations and associated tumors. For these reasons, clinicians recommend individualized therapy, taking into consideration the clinical conditions of the patient and potential adverse effects of the immunosuppressive drugs.
PNP management involves a careful and multidisciplinary approach aiming to adequately treat the underlying neoplasm and control the mucocutaneous lesions.69,70 An ophthalmological evaluation is essential to determine the degree of ocular involvement and the appropriate treatment in order to prevent blindness. Corticosteroid eye drops, lubricants, and artificial tears are often required. Amniotic membrane graft is an alternative in severe cases with conjunctival and fornix scarring.34,36
One of the key elements of PNP treatment lies in the complete excision of the concurrent solid tumor or control of the associated hematologic neoplasia. Tumoral cells may produce autoantibodies able to recognize epidermal antigens involved in the pathogenesis of PNP, thus contributing to the development of the paraneoplastic manifestations.63,34 PNP and tumor activity may follow a parallel course and recur simultaneously;25 however, there are reports of independent progression.32,47 In addition, antibodies directed against tumoral antigens may cross-react with desmosomal and hemidesmosomal glycoproteins and trigger a disruption of mucocutaneous integrity.40
An overexpression of IL-6 by neoplastic cells may also promote a dysregulation of the immune response with inhibition of T-regulatory cells and stimulation of cytotoxic T cells, further exacerbating the tissue damage.27 Improvement of cutaneous and mucosal lesions of patients after the removal of thymoma and Castleman’s tumor provided additional evidence of the importance of adequate control of the underlying neoplasia as part of PNP treatment.25
Successful management of cutaneous lesions with systemic steroids such as prednisone 1.0-1.5mg/kg/day or in pulse therapy is possible, but mucosal improvement rarely occurs in monotherapy.70 Options for treatment of recalcitrant mucous involvement include a combination of adjuvant immunosuppressive agents and immunobiologics with unpredictable and variable responses.
As an unbalanced T- and B-cell immune response participates in the development of PNP, immunosuppressive drugs that target both immune mechanisms have been employed in an attempt to control the disease.40,64 Azathioprine is metabolized into 6-mercaptopurine (an active drug) that is incorporated into the cell DNA and antagonizes the synthesis of nucleic acids and proteins.71,72 This results in cell cycle arrest and death, thus inhibiting lymphocyte proliferation and autoantibody production.73 Mycophenolate mofetil is a prodrug of mycophenolic acid that inhibits de novo synthesis of guanine nucleotides, halting T- and B-cell proliferation.74
The cytotoxic effects of azathioprine and mycophenolate mofetil on both humoral and cell-mediated responses are useful to reduce autoantibody synthesis and deplete autoreactive immune cells that compose the inflammatory infiltrate observed in PNP lesions, such as CD8+, CD56+, and CD68+ cells.64 Cyclosporin is a calcineurin inhibitor able to reduce T-lymphocyte activation and IL-2 production.10 The use of cyclosporine 5-7mg/kg/day may improve cutaneous lesions, but with variable response of mucous involvement.75,76 Furthermore, the risk of B-cell proliferation and nephrotoxicity induced by cyclosporine has to be considered before the introduction of this drug.10 Williams et al. described the successful use of mycophenolate mofetil and azathioprine in a 40 year-old female patient who developed PNP two years after the diagnosis and treatment of chronic lymphocytic leukemia. Initial therapy with prednisone, azathioprine, and cyclosporine improved only the cutaneous lesions, while recalcitrant oral erosions led to a body weight loss of 20%. Cyclosporin was then replaced by mycophenolate mofetil, with gradual improvement of oral lesions that were completely cleared after ten months.74
With the advent of immunobiologics, targeted therapy in PNP has emerged as a new treatment option aiming towards increased antigen specificity and fewer adverse events. Rituximab is an immunobiologic medication composed of chimeric anti-CD20 monoclonal antibodies that target both normal and tumoral cells expressing CD20.77 Therefore, it is an interesting therapeutic option to treat underlying CD20+ lymphoproliferative disorders, in addition to controlling the paraneoplastic autoimmunity.78 Nevertheless, patients exhibit an inconsistent response to rituximab, with reports of both successful control of the PNP lesions and poor response, and even PNP onset within six months to 11 years after treatment of hematologic malignancies with rituximab.45,78-83 Some authors advocate that early treatment with rituximab may improve drug efficacy and prevent PNP refractoriness.77 It has also been postulated that CD20 lymphocytes and tumoral cells might be responsible for maintenance of autoantibody production, thus contributing to persistence of disease activity.80 Additional studies are necessary to confirm these theories.
Another drug that targets both T and B lymphocytes is alemtuzumab. This medication, produced from monoclonal anti--CD52 IgG1 antibodies, has been used in the treatment of lymphoproliferative disorders (dose of 30mg subcutaneously three times a week) to induce cell death and prolonged lymphopenia.84 To date, five patients with B cell lymphoma and PNP were treated with alemtuzumab in combination with prednisone and intravenous immunoglobulin. Due to increased risk of infection, patients also received antimicrobial prophylaxis with acyclovir, sulfamethoxazol/ trimethoprim, and fluconazole.84,85 Mucocutaneous lesions started improving within two weeks and achieved complete remission after 6-12 weeks. However, infectious complications led to death in four patients after treatment with alemtuzumab: in three patients concomitant PNP recurrence occurred 2.5-5 years after anti-CD52 therapy and in one patient PNP was still under remission after two years when she died due to an invasive fungal infection.44,85 The only patient reported to be alive and under remission with prednisone and mycophenolate mofetil had a shorter follow-up of one year.84
As autoantibodies play an important role in the pathogenesis of PNP, a rapid depletion of circulating autoreactive immunoglobulins with plasmapheresis has been used to achieve disease control.86 Kitagawa et al. reported a 67-year-old female patient with clinical and immunopathological features of PNP, including reactivity with envoplakin and periplakin with immunoblotting, though no underlying neoplasm was identified. She presented extensive oral mucosal erosions and dyspnea with hypoxemia that were refractory to pulse therapy with methylprednisolone, cyclosporine, and intravenous immunoglobulin. Treatment with plasmapheresis was initiated and after four sessions the mucocutaneous lesions improved. Nevertheless, worsening of the bronchiolitis obliterans led to respiratory failure and death after nine months.60 Sustained remission following plasmapheresis may be obtained with adjuvant treatment with cyclophosphamide to avoid the synthesis of autoantibodies and subsequent increase in the circulating levels that induce disease recurrence.77
Intravenous immunoglobulin (IVIg) is an alternative to counteract and decrease circulating pathogenic autoantibodies, and to reduce inflammation through modulation of cytokines, complement, endothelial cells, and lymphocytes.25 Another advantage is the lack of immunosuppressive effect of IVIg, thus increasing the safety of this therapy, especially in combination with other immunosuppressants.39 IVIg has been used to treat cutaneous lesions as well as bronchiolitis obliterans, which is one of the main causes of death in PNP.32 In a retrospective case series published by Wang et al., six out of ten patients with PNP associated with Castleman’s tumor received 10-20 g of IVIg preoperatively (n = 1), and maintained the infusions during surgical removal of the neoplasia (n = 6), and postoperatively (n = 2). All patients who received IVIg started improving within three days after tumor resection, whereas three out of four patients who refused to receive IVIg treatment developed bronchiolitis obliterans within one week of postoperative follow-up.25
Final ConsiderationsPNP is a challenging autoimmune paraneoplastic syndrome, both in terms of diagnosis and treatment. A multitude of clinical presentations with polymorphic lesions that resemble erythema multiforme, lichen planus, or pemphigus vulgaris may delay diagnostic suspicion and confirmation. Refractory lesions – especially involving the oral mucosa – should prompt a systemic workup to rule out a hematologic or solid concomitant neoplasia. Recent advances in PNP, with the discovery of new antigens involved in the pathogenesis of the disease, may increase the accuracy in diagnosis of PNP and also provide evidence for targeted therapy.
AcknowledgmentsThe authors wish to thank Dr. Maria Vitoria Quaresma, a member of the dermatopathology team of the Hospital das Clínicas of the Faculdade de Medicina of USP, who kindly prepared the histologic images used in this study.
Questions- 1.
What are the main histopathological findings in paraneoplastic pemphigus?
- a)
Vacuolar degeneration of the basal layer with lichenoid inflammatory infiltrate;
- b)
Apoptotic keratinocytes with interface vacuolar dermatitis;
- c)
Suprabasal acantholysis with lichenoid interface dermatitis;
- d)
Subepidermal detachment with lichenoid inflammatory infiltrate.
- a)
- 2.
The neoplasia most commonly associated with paraneo-plastic pemphigus in children is:
- a)
Thymoma;
- b)
Lymphoma;
- c)
Acute lymphocytic leukemia;
- d)
Castleman disease.
- a)
- 3.
Paraneoplastic pemphigus is clinically characterized by:
- a)
Polymorphic cutaneous lesions refractory to treatment, without mucous or systemic involvement;
- b)
Polymorphic mucocutaneous lesions refractory to treatment, with systemic involvement;
- c)
Mucositis refractory to treatment and polymorphic cutaneous lesions, without systemic involvement;
- d)
Polymorphic mucocutaneous lesions responsive to treatment, with systemic involvement.
- a)
- 4.
What is the treatment currently recommended to manage paraneoplastic pemphigus?
- a)
Treatment of the underlying neoplasia, without systemic therapy;
- b)
Treatment of the underlying neoplasia and systemic corticosteroid with or without immunosuppressive therapy;
- c)
Treatment of the underlying neoplasia and systemic corticosteroid without immunosuppressive therapy;
- d)
Treatment of the underlying neoplasia and immunosuppressive therapy.
- a)
- 5.
The most specific antigens recognized by autoantibodies in paraneoplastic pemphigus are:
- a)
Desmocollin 2 and envoplakin;
- b)
Desmoglein 3 and BP180;
- c)
Desmoglein 1 and BP230;
- d)
Periplakin and desmoplakin.
- a)
- 6.
What are the main causes of mortality in paraneoplastic pemphigus?
- a)
Bronchiolitis obliterans and sepsis;
- b)
Sepsis and pulmonary thromboembolism;
- c)
Multiple organ failure and metastases;
- d)
Acute respiratory distress syndrome and myasthenia gravis.
- a)
- 7.
According to the current knowledge about the pathogenesis of paraneoplastic pemphigus, it is possible to affirm that:
- a)
The cytotoxic immune response is related to the presence of lichenoid lesions similar to erythema multiforme, with or without circulating autoantibodies;
- b)
The humoral immune response triggers polymorphic mucosal lesions, which may be lichenoid or bullous;
- c)
The cytotoxic immune response is responsible for the development of bullous mucocutaneous lesions;
- d)
The humoral immune response correlates with the presence of exclusive mucosal blistering.
- a)
- 8.
What are the main neoplasias related to the development of paraneoplastic pemphigus?
- a)
Hodgkin’s lymphoma, breast carcinoma, thymoma;
- b)
Chronic lymphocytic leukemia, Hodgkin’s lymphoma, Castleman disease;
- c)
Non-Hodgkin lymphoma, Waldeström macroglobulinemia, monoclonal gammopathy;
- d)
All the above mentioned.
- a)
- 9.
The diagnosis of paraneoplastic pemphigus usually:
- a)
Precedes the diagnosis of the underlying neoplasia;
- b)
Follows the diagnosis of the underlying neoplasia;
- c)
Correlates with the recurrence of the underlying neoplasia;
- d)
Has no temporal correlation with the underlying neoplasia.
- a)
- 10.
The following laboratorial evaluation is considered specific for the diagnosis of paraneoplastic pemphigus:
- a)
Biopsy of a cutaneous lesions for histopathological evaluation, immunohistochemistry, and serum sample for indirect immunofluorescence with human foreskin as substrate;
- b)
Biopsy of a mucous lesion for direct immunofluorescence study, serum sample for indirect immunofluorescence using human foreskin as substrate;
- c)
Biopsy of a cutaneous lesion for direct immunofluorescence study, serum sample for ELISA anti-desmoglein, and indirect immunofluorescence using murine transitional epithelium as substrate;
- d)
Mucocutaneous biopsy for histopathological and direct immunofluorescence evaluation, serum sample for indirect immunofluorescence using murine transitional epithelium as substrate.
- a)
Answers
Pemphigus vulgaris. An Bras Dermatol. 2019;94(3):264-78.
1. C
2. D
3. C
4. D
5. D
6. C
7. B
8. C
9. C
10. B