Psoriasis is a chronic and prevalent disease, and the associated pruritus is a common, difficult-to-control symptom. The mediators involved in psoriatic pruritus have not been fully established.
Objective:To evaluate associations between the number of mast cells in psoriatic lesions and the intensity of pruritus.
Methods:29 patients with plaque psoriasis were recruited. In all participants, Psoriasis Area and Severity Index and Body Surface Area were assessed. A questionnaire was administered to obtain clinical information and the Dermatology Life Quality Index. Pruritus was assessed using a visual analog scale and skin biopsies were performed for staining with Giemsa and Immunohistochemistry with C-Kit.
Results:Pruritus was observed in 91.3% of our patients. Median VAS was 6 (p25-75: 2-8). The immunohistochemical method revealed a mean of 11.32 mast cells/field and Giemsa staining revealed a mean of 6.72 mast cells/field. There was no correlation between the intensity of pruritus and mast cell count, neither in Immunohistochemistry (p = 0.15; rho = -0.27) nor in Giemsa (p = 0.16; rho = -0.27). Pruritus did not impact on the Dermatology Life Quality Index (p = 0.51; rho = -0.13).
Study limitations:The small sample size may be considered the main limitation of our study.
Conclusions:Although mast cells are mediators of pruritus in many cutaneous diseases, our findings support that psoriatic pruritus is a complex disorder with multifactorial, complex pathophysiology, involving pruritogenic mediators others than mast cells.
Psoriasis is a chronic, inflammatory and immune-mediated condition, with a prevalence of 1-3% in Western countries.1,2 Pruritus is seen in 70% to 90% of patients with psoriasis.3-6 Despite being a very frequent symptom, pathophysiology of pruritus in psoriasis it is not yet understood.7 Many studies demonstrate increased expression of substance P in psoriasis plaques, being this substance also implicated in the inflammatory and proliferative process of other skin conditions.8
The presence of mast cells in established plaques and in early lesions of psoriasis has been documented in many studies, with the increase in number and the early activation of cutaneous mast cells typical findings of psoriatic inflammation.9-13 One of the early morphological changes observed is mast cell degranulation.14,15 In active lesions and in nonaffected skin of patients with psoriasis, mast cell degranulation is associated to vascular changes and angiogenesis.14,16 Both phenomena are vitally important in the pathophysiology of psoriasis.17
Cutaneous innervation is increased in the skin with psoriasis and pruritus compared to the skin with no pruritus.18 The increased innervation can worsen the intensity of the symptom in psoriasis patients with inflammation,19 what can explain the higher incidence of pruritus in the phases when the disease is more active. Changes in dermal vasculature secondary to the release of histamine by mast cells, in psoriasis lesions can maybe have an important role in the pathophysiology of pruritus in psoriasis.7
The majority of patients incriminates stress as a triggering factor for both psoriasis lesions and the pruritus associated to the condition.3,18 The mechanism by which stress triggers pruritus in psoriasis is not clear, however, it is known that emotional stress changes the levels of certain neuropeptides like substance P, either in the CNS or in the tissues.20 Substance P, besides being one of the most potent endogenous pruritogenic peptides, when increased in situations of stress, can lead to mast cell degranulation.20,21
There are few studies regarding the quantification of mast cells in psoriasis lesions. This is one of the few studies that proposes to evaluate the relationship between the amount of mast cells in psoriasis lesions with the intensity of the pruritus.
MethodsIt is a cross-sectional study design, conducted from April to December 2013. During this period, patients 18 years of age or older, with psoriasis vulgaris who were patients at the ambulatory of psoriasis of the service of dermatology at Hospital de Clínicas de Porto Alegre were invited to participate in the study. The sampling was performed by convenience, with 29 patients included in the study after the eligibility criteria. Were considered exclusion criteria: patients taking medications that induce mast cell degranulation, pregnancy and patients being treated with phototherapy.
The research was approved by the ethics committee in research of the Universidade Federal do Rio Grande do Sul under the protocol number 13-0128. All patients included in the study signed an informed consent form.
Patients filled out questionnaires: one for the evaluation of the clinical and epidemiological characteristics such as sex, age, time of evolution of psoriasis, family history of psoriasis, comorbidities, use of medications, alcohol intake and smoking; the other was to assess the impact of psoriasis in the quality of life through the DLQI. The intensity of the pruritus in the last 24 hours was estimated with the VAS, and the PASI and BSA with the dermatological examination.
After clinical evaluation, the patient underwent a 4mm punch skin biopsy in an area with an active lesion of psoriasis. Skin samples were fixated in formalin, embedded into paraffin blocks and stained with GIEMSA and C-Kit (CD-117). Mast cell density was analyzed by a single pathologist on the optical microscopy with a 40x power. At least three fields were examined and the main number of mast cells calculated for each of the two staining methods.
The collected data were inserted into an Excel 2007 spreadsheet, and statistical tests were performed with the program SPSS version 18.0. The comparison of symmetrical quantitative variables was performed with the Student’s t-test or ANOVA test, depending of the categories evaluated, and were correlated by Spearman’s correlation coefficient. Those with asymmetrical distribution were compared by the Mann-Whitney test and the categorical by the Fisher’s exact test. The concordance between the staining methods for the evaluation of mast cells was performed by the Bland and Altman technique and evaluated by the intraclass correlation coefficient (ICC) for perfect concordance (random two-way).22 A level of significance of 5% was considered.
ResultsClinical and epidemiological characteristicsOf the 29 patients participating in the study, 44.8% were male and 55.2% female, with a mean age of 50 years. Of those, 89.7% were Caucasian. Median time of the disease was of 18 years (Ir 11-30.5) and family history of psoriasis was present in 37.9% of those studied.
Most patients (65.5%) had been using some type of medication for the treatment of psoriasis: 20.7% were using methotrexate (MTX), 6.9% acitretin, 58.6% topical steroids and 17.2% formulations with liquor carbonis detergens (LCD) and salicylic acid (SA).
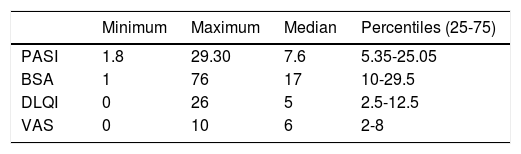
The population of the study was very diverse in regards to PASI, BSA, DLQI and VAS. According to what is shown on table 1, we can observe that the median PASI was 7.6 (p25-75: 5.35-25.05), DLQI was 5 (p25-75: 2.5-12.5) and VAS was 6 (p25-75: 2-8).
Pruritus featuresPruritus was reported by 93.1% of patients, 51.7% of those with daily symptoms. Most (82.8%) complained of the symptoms only in the psoriasis lesions while 17.2% complained of diffuse pruritus. Alleviating factors were reported by 75.9% of patients, the most common being the use of topical steroids (24.1%) and cutaneous hydration (48.2%). Among the triggering factors, reported and 72.4% of cases, the most frequent were stress/anxiety (27.6%) and exposure to heat (27.6%). Showering was described as an alleviating factor for the pruritus of eight patients (27.6%) and as a triggering factor by three (10.3%).
Evaluation of skin biopsiesOf a total of 58 slides evaluated, 29 stained with GIEMSA and 29 with C-Kit, a mean mast cell count of 6.3 was obtained (p25-75: 4.9-8) and 10.7 (p25-75: 8.65-13.5) for GIEMSA and immunohistochemistry, respectively.
We did not find any statistically significant correlation in the quantification of mast cells between PASI scores (p= 0.718 and rho= -0.070 IHC; p= 0.314 and rho= 0.194 GIEMSA), DLQI (p= 0.692 and rho= -0.077 IHC; p= 0.769 and rho= 0.057 GIEMSA), VAS (p= 0.152 and rho= -0.273 IHC; p= 0.159 and rho= -0.269 GIEMSA).
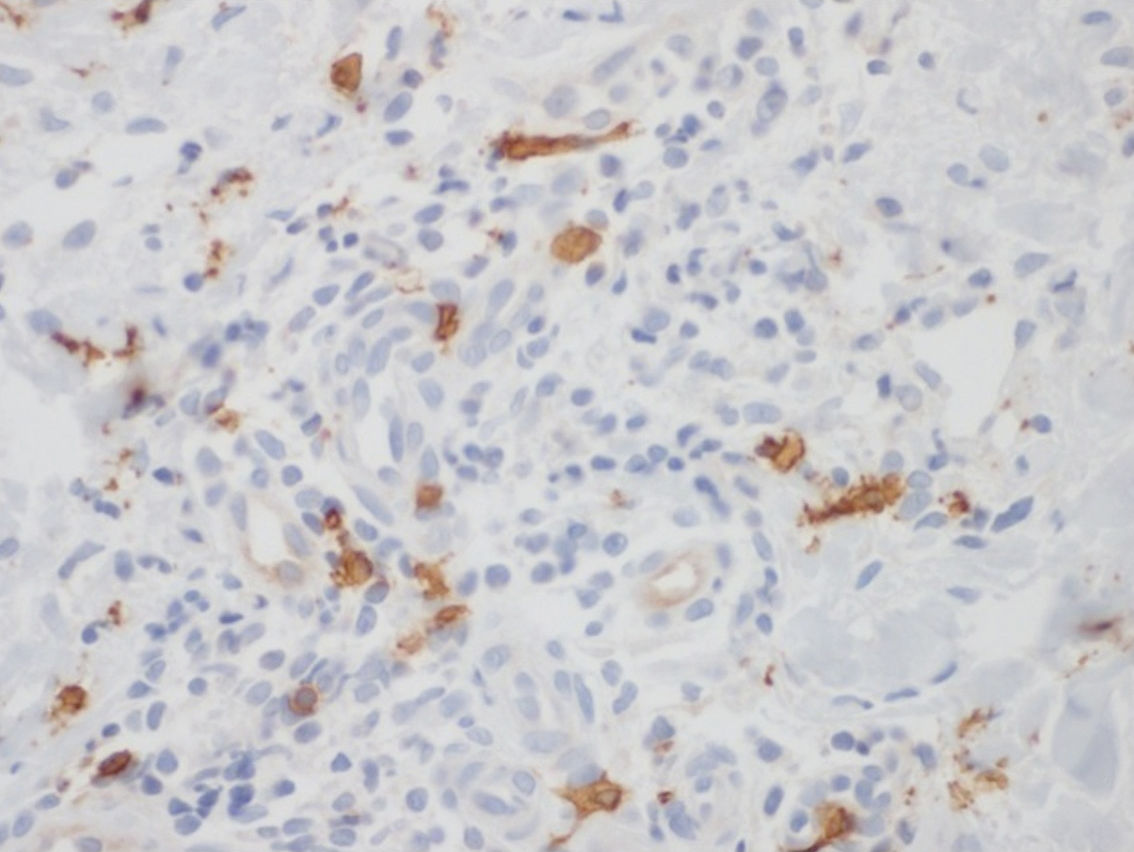
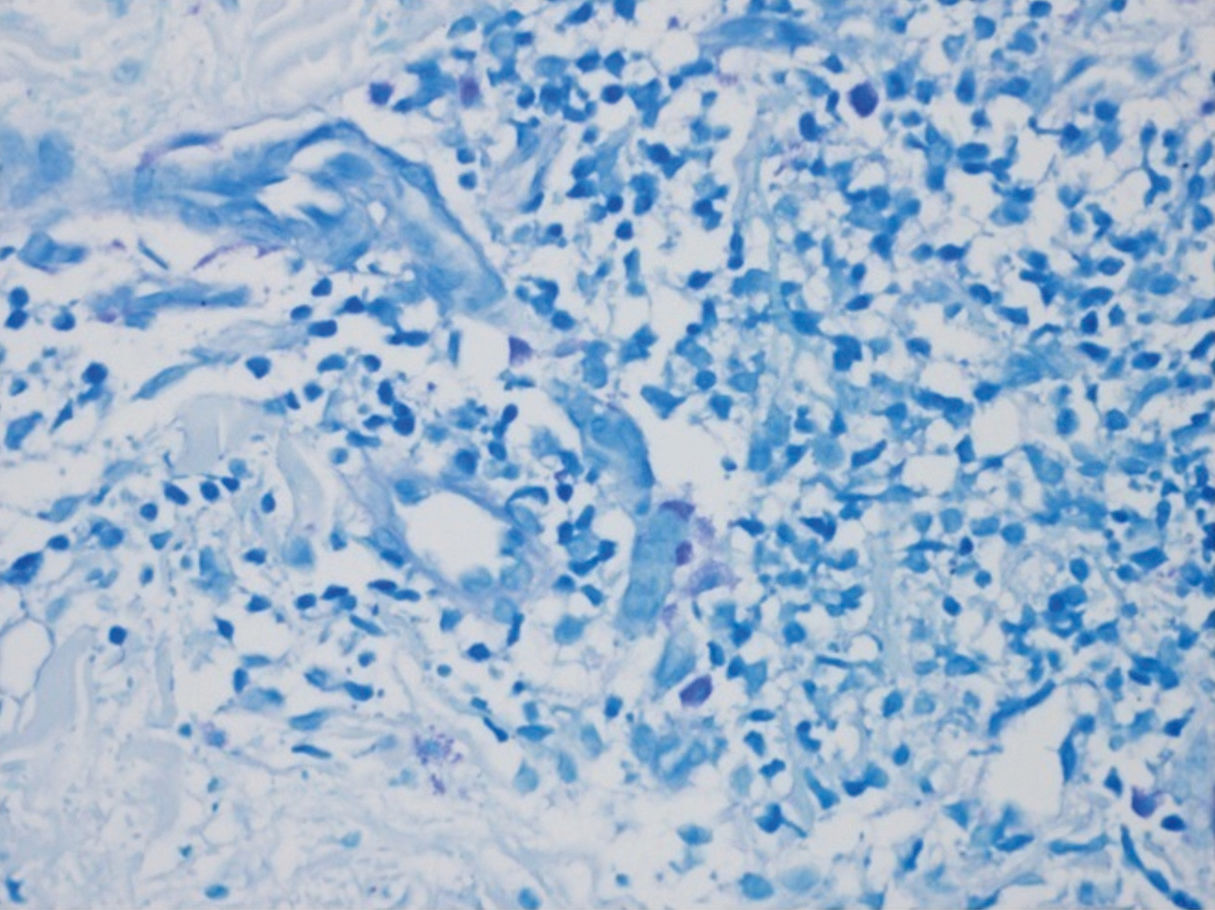
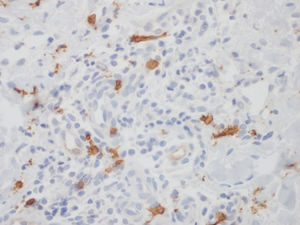
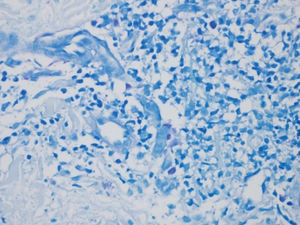
The larger number of mast cells (mean of 21.6 mast cells between fields stained by IHC – figure 1 – and 10 mast cells with GIEMSA – figure 2) was seen in a patient with mild psoriasis (PASI= 5.3 and BSA=1) with no complaints of pruritus (VAS=0).
The most severe psoriasis patient in our study (PASI= 29.3 and BSA=76) had a VAS=7 and the mean amount of mast cells in their skin biopsy was of 14.6 and 9.64 IHC and GIEMSA, respectively.
The analysis of the quantification of cells in relation to the sex found for men a mean of 6.9 (standard deviation = 3.3) mast cells in GIEMSA and for women a mean of 6.4 cells (standard deviation = 1.9), with no statistically significant difference between those values (p = 0.602). The mean number of mast cells evaluated by IHC was 12.8 (standard deviation = 5.5) for men and 10.2 (standard deviation = 3.1) for women, also with no statistically significant difference.
In table 2, we compared the density of mast cells (mean variable for both stains – IHC and GIEMSA) in patients who were using some treatment for psoriasis (topical or systemic) and those who were not using any treatment at the time of the study, with no statistically significant difference between those. Even though patients using mast cell degranulating drugs were excluded from the study, the use of drugs for the treatment of psoriasis could have altered the mast cell count in the lesions of psoriasis, as well as the assessment of pruritus of these patients.
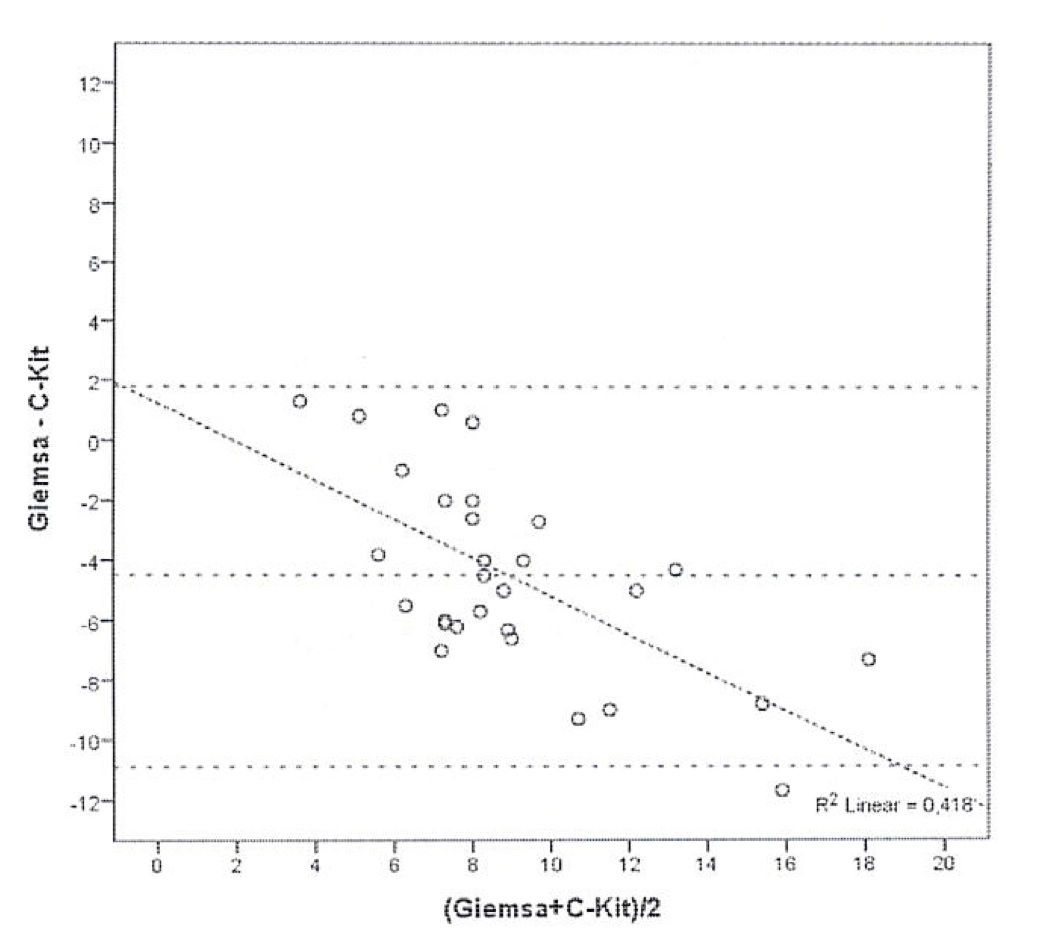
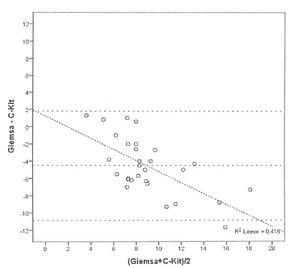
Bland and Altman’s graph shows the mean of the differences and the limits with a dotted line (figure 3). The limits of 95% of the concordance of the two techniques of quantification of mast cells between -1.49 and 10.83. C-Kit staining identifies more mast cells than GIEMSA, and as the number grows, the difference between mast cells increase. After calculating the intraclass coefficient (ICC = 0.344) for mast cell counts with GIEMSA and C-Kit, we can observe in figure 3 that C-Kit count increases compared to GIEMSA according to the increase in the number of cells. After 6-7 cells, the difference is more than five cells, what could support the use of C-Kit instead of GIEMSA as the preferred technique for the quantification of mast cells in the dermis.
DiscussionPsoriasis is a chronic, stigmatizing dermatological disease of difficult management, many times with systemic involvement and negative impact on the quality of life of the patients. Pruritus is a frequent symptom; however, it is many times underestimated in patients with psoriasis, contributing even further to reduce quality of life of those affected.23
Bilac et al24 assessed 87 patients with psoriasis and found a higher frequency of pruritus in patients with DLQI≥10. Different to these findings, we did not find an association between pruritus and quality of life in the population of our study.
Our results confirm data in the literature that demonstrate that pruritus is one of the most prevalent symptoms in patients with plaque psoriasis.18,24 As Amatya et Nordlind25 and Nakamura et al.,19 we did not find a relationship between pruritus and psoriasis severity, even though this association was reported by other authors.5 With similar results, Prignano et al.18 did not identify a statistically significant correlation between the presence and intensity of pruritus with the patient’s age, sex, and psoriasis severity according to PASI scores either.
For the adequate management of pruritus in psoriasis, it is necessary to understand its pathophysiology. Nakamura et al.19 were the first to document the local market is associated to pruritus in psoriasis through histological examination of the lesions of 38 symptomatic patients compared to the findings in asymptomatic patients. The peculiar feature of finding mast cells in lesions of patients with pruritus was the presence of granules closely juxtaposed to the perineurium of non-myelinated nerve fibers, finding that was not seen in any patient without pruritus. Contrary to our findings, the authors observed the large amount of degranulating mast cells in the papillary dermis of patients with pruritus. However, in the above-mentioned study, the patients discontinued their psoriasis topical treatments two weeks before starting the study, and the systemic treatments three months before. In our study, both topical and systemic treatment for psoriasis were maintained, representing a possible limitation, because it is known that the use of topical steroids and some systemic medications such as retinoids in cyclosporine can reduce the mast cell count.26
The major limitation of our study was the small sample size, with an estimated calculation in order to detect a moderate correlation with a value of r≥ 0.5, considering a strength of 80% and the significance level of 0.05. However, it is also important to note that we did not consider the time of the lesions or the biopsy site (if in a photoexposed area or not) in our results. These data could alter our results, since a higher density of mast cells can be seen in recent lesions of psoriasis and in photoexposed areas since mast cells are activated and recruited by ultraviolet and infrared radiation, as well as by heat.9,27
Toruniowa e Jablónska9 studied 122 patients with psoriasis and 80 controls and showed an increase in the number of mast cells after trauma on the skin, either from patients with psoriasis or controls, demonstrating an important role of the mast cells in the inflammatory response of the skin to trauma. Moreover, they proved that the Koebner phenomenon is associated to the buildup of mast cells, reinforcing the involvement of mast cells in early psoriasis lesions.
Multiple mediators have been associated to pruritus in psoriasis, but none was in fact proven as cause of pruritus. Histamine, which is one of the main pruritus mediators in many dermatological conditions, seems not to be involved in the symptom of patients with psoriasis. A correlation between the intensity of the pruritus and serum levels of histamine in patients with psoriasis has not been demonstrated, as well as no difference in serum levels of histamine between psoriasis patients, with and without pruritus.28
ConclusionMany studies already demonstrated the abnormal expression and/or distribution of many neuropeptides and their receptors in psoriasis lesions, among those substance P (SP) calcitonin gene-related peptide (CGRP) and vasoactive intestinal peptide (VIP). Besides, many cytokines, in particular IL-2 have also been implicated in the pathophysiology of pruritus in psoriasis.19 However, there are still many gaps, such as the exact role of the central nervous system in chronic pruritus. Therefore, more studies are needed to better understand the pathophysiological mechanisms of pruritus and psoriasis.
Despite acting as important inflammatory cells in the pathophysiology of psoriasis, we did not find a role for mast cells in the pruritus of psoriasis in our study, what reinforces the concept that the pathophysiology of pruritus in psoriasis is complex and multifactorial. We also conclude that the estimated mast cell count is more precise with the technique of IHC compared to GIEMSA, especially when a large number of mast cells is considered. Future studies are needed, evaluating the pruritus mediators and considering other variables, such as time of the lesions and areas biopsied for better understanding of pruritus in psoriasis.
Financial support: Fundo de Incentivo à Pesquisa do HCPA (FIPE) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ).
Conflict of interest: None.