Children’s products are considered safe by the general population and doctors. Labels with terms such as “hypoallergenic” or “dermatologically recommended and tested” denote trust and credibility with the idea that they can be used by any individual. Patients with allergic contact dermatitis may be sensitive to allergens present in any product, including children’s. There is insufficient knowledge about allergens in these products in our country. We evaluated 254 children’s products, and at least one allergen was present in 236 (93%) of them. The indication of a topical product should be careful and based on contact tests.
Skin care products for children are considered safe by the general population and physicians. Many carry on the label terms such as “hypoallergenic” or “recommended and tested by a dermatologist”, conferring reliability and credibility to the idea that they can be used by any individual. However, it is known that allergic contact dermatitis (ACD) patients can be sensitive to allergens in any product, including children’s products.
ACD can occur at any point in life, including childhood. Even though the lesions can appear in sites with intact skin, there is a higher risk in areas with previous skin conditions and with abnormal cutaneous barrier. Besides, continuous use of the product is another facilitator for ACD.1
Children’s cosmetic products are popular in our society. The Brazilian market is one of the largest in this segment, regulated by ANVISA (Agência Nacional de Vigilância Sanitária).2
The term hypoallergenic is a matter of debate among specialists. It is applied to cosmetics, hair dyes and jewelry, and means that the product has a low ability to promote, trigger or boost cutaneous reactions.3 In our society, products using this term undergo clinical tests of cutaneous sensitization and photoallergy, which verify a low incidence of adverse reactions.4
The lack of knowledge on the presence of allergens in children’s products in our society motivated this study. Between September and October 2016, we evaluated in the city of São Paulo products found in the children’s market through information on the packages or on the manufacturer’swebsite, and that was the only inclusion criterion.
The evaluated products belonged to the following groups: shampoos, conditioners, soaps, perfumes, nappy rash creams, moisturizers, oils, repellents, powders, wet wipes, and make ups.
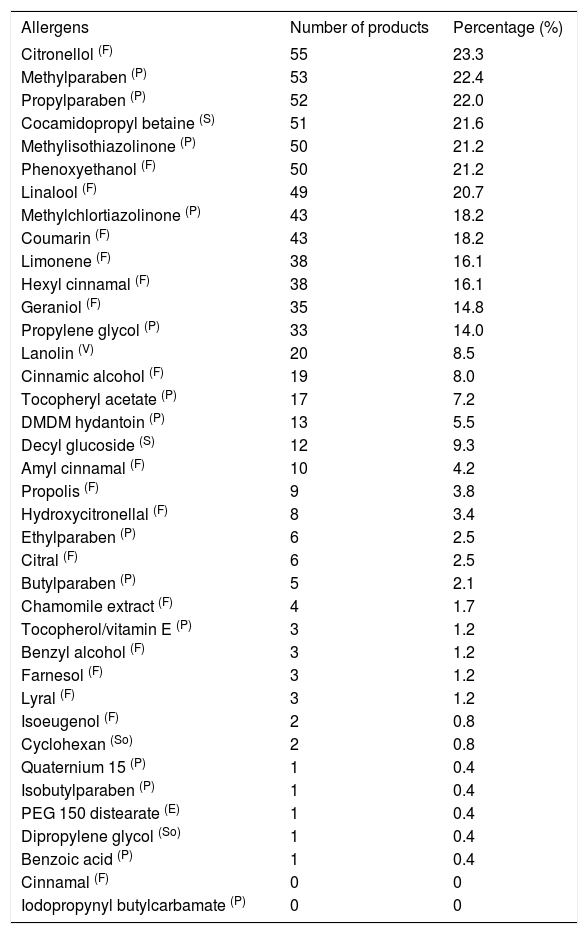
We evaluated 254 products of personal hygiene and perfumes of different brands for the presence of 38 substances considered allergens, such as fragrance components, preservatives, emulsifiers, solvents, surfactants and vehicles, shown in table 1.
Allergens present in 236 children’s products
| Allergens | Number of products | Percentage (%) |
|---|---|---|
| Citronellol (F) | 55 | 23.3 |
| Methylparaben (P) | 53 | 22.4 |
| Propylparaben (P) | 52 | 22.0 |
| Cocamidopropyl betaine (S) | 51 | 21.6 |
| Methylisothiazolinone (P) | 50 | 21.2 |
| Phenoxyethanol (F) | 50 | 21.2 |
| Linalool (F) | 49 | 20.7 |
| Methylchlortiazolinone (P) | 43 | 18.2 |
| Coumarin (F) | 43 | 18.2 |
| Limonene (F) | 38 | 16.1 |
| Hexyl cinnamal (F) | 38 | 16.1 |
| Geraniol (F) | 35 | 14.8 |
| Propylene glycol (P) | 33 | 14.0 |
| Lanolin (V) | 20 | 8.5 |
| Cinnamic alcohol (F) | 19 | 8.0 |
| Tocopheryl acetate (P) | 17 | 7.2 |
| DMDM hydantoin (P) | 13 | 5.5 |
| Decyl glucoside (S) | 12 | 9.3 |
| Amyl cinnamal (F) | 10 | 4.2 |
| Propolis (F) | 9 | 3.8 |
| Hydroxycitronellal (F) | 8 | 3.4 |
| Ethylparaben (P) | 6 | 2.5 |
| Citral (F) | 6 | 2.5 |
| Butylparaben (P) | 5 | 2.1 |
| Chamomile extract (F) | 4 | 1.7 |
| Tocopherol/vitamin E (P) | 3 | 1.2 |
| Benzyl alcohol (F) | 3 | 1.2 |
| Farnesol (F) | 3 | 1.2 |
| Lyral (F) | 3 | 1.2 |
| Isoeugenol (F) | 2 | 0.8 |
| Cyclohexan (So) | 2 | 0.8 |
| Quaternium 15 (P) | 1 | 0.4 |
| Isobutylparaben (P) | 1 | 0.4 |
| PEG 150 distearate (E) | 1 | 0.4 |
| Dipropylene glycol (So) | 1 | 0.4 |
| Benzoic acid (P) | 1 | 0.4 |
| Cinnamal (F) | 0 | 0 |
| Iodopropynyl butylcarbamate (P) | 0 | 0 |
P = preservative; F = fragrance; S = surfactant; E = emulsifier; V = vehicle; So = solvent
Data were collected and added into an Excel® spreadsheet, analyzed and compared to the ones in the literature.
In the products’ packages there was information such as: “dermatologically tested” (121; 43.8%), “hypoallergenic” (99; 35%), “minimizes allergies” (15; 5.3%) and “safe/harmless” (18; 6.4%), and no information was present in 30 (10%). In some packages, there was more than one term.
Among the products analyzed, 236 (93%) had at least 1 allergen: 62 products with 1 allergen (24.4%), 51 with 2 (20%), 26 with 3 (10.2%) and 97 with more than 4 (38.3%). Seventeen products did not show any of the evaluated substances (7.1%).
Among the 38 allergens evaluated, 36 (94.7%) were present: 17 components of fragrances (47.5%), 13 preservatives (36.1%), two surfactants (5.5%), two solvents (5.5%), one emulsifier and one vehicle (2.7% each). Cinnamal and iodopropynyl butylcarbamate were not found in any product. A publication from 2017 also showed fragrances as the main allergen in Brazilian products.5
Of all the allergens found, the most prevalent was citronellol (55; 23.3%), used in fragrances. It is a component of rose and geranium oils, part of fragrance mix II (alpha-hexyl-cinnamal, coumarin, farnesol, lyral, citral, citronellol) in contact tests. Although not used in contact tests in our country, it is a potential allergen to be investigated, considering its frequency among the products studied. Fragrances are present in perfumes, cosmetics, topical medications, personal hygiene, and cleaning products and are a common cause of ACD. However, in general, product labels do not specify, as required by the ANVISA’s resolution # 16/2011, what would help guiding patients.
Preservatives, including parabens, are used in cosmetics and food. The most common esters are methylparaben, propylparaben, ethylparabes and butylparaben. The frequency of ACD caused by this group of preservatives is low in different centers (from 0.3% to 3.5%), and some authors suggest removing them from standard contact test series.6,7 In our service, unpublished data show 19/1340 (1.4%) positive tests to the paraben group in the period between 2011 and 2016, supporting the above mentioned observations.
Methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) were found in 21.2% and 18.2 % of the products, respectively, while Harmann et al. found them in 10%.8 This observation is relevant and must be highlighted since they are allergens that cause severe contact dermatitis, either in adults and children, whose sensitization frequencies have increased throughout the world and in our country.9
Cocamidopropyl betaine is a surfactant in soaps, shampoos, bubble bath, and toothpastes that thickens and softens the foam and is not irritant to the user’s eyes. In our study, it was present in 51 products (21.6%). In a study published in 2015 assessing 187 children’s products, it was the most prevalent allergen. In childhood ACD studies, it ranks among the 10 most important allergens.8,10
Children’s products are commonly labeled as hypoallergenic and considered safe to be used by any individual. However, as we demonstrated here, this is not the case, since among the 254 personal hygiene products studied, 236 (93%) had at least one allergen in its formulation.
Many of the allergens present in the products are not in the patch test series available in our country, what should be reassessed by expert groups.
Therefore, this is an alert for the authorities and physicians. Components described in the labels should be evaluated before the product is considered safe for certain individuals, particularly those with ACD and atopic dermatitis. In these cases, the indication of the products should be guided by contact tests.
Received 13 July 2017.
Accepted 30 August 2017.