The 2024 Brazilian Consensus on Psoriasis and Treatment Algorithm of the Brazilian Society of Dermatology (SBD, Sociedade Brasileira de Dermatologia) comes four years after the release of the last Consensus, published in 2020. This new consensus now includes 43 chapters and has the participation of 85 professionals from across Brazil, including dermatologists and rheumatologists working in university hospitals, research centers, and public and private healthcare systems. The concept of Cumulative Life Course Impairment (CLCI), the inclusion of new biologics approved in Brazil after 2020, the update on psoriasis treatment in specific situations (such as pregnancy and surgery), and the discussion on "Response Predictors" are examples of updates addressed in this Consensus. The updated treatment algorithm used experts’ responses through the Delphi method and, among other modifications, removed acitretin from the plaque psoriasis treatment sequence, defined treatment lines for choosing immunobiologicals in both adult and pediatric populations, and included spesolimab and cyclosporine as first-line treatments for generalized pustular psoriasis. Finally, the new Consensus addresses the place of small molecule inhibitors in the algorithm and discusses future treatment options for plaque psoriasis.
The Brazilian Consensus on Psoriasis 2024 and Treatment Algorithm of the Brazilian Society of Dermatology (SBD) comes four years after the release of the last "Brazilian Consensus on Psoriasis 2020,"1 a period during which significant advances and innovations in the study of psoriasis were made, making updating the topic essential.
To develop this new work,2 which now includes 43 chapters, the authors relied on the participation of 85 experts from across Brazil, including dermatologists and rheumatologists from a wide range of regions (Fig. 1) and working in university hospitals, research centers, and public and private healthcare systems.
Among the updates to the 2024 Consensus, the authors highlight the introduction of the concept of CLCI (Cumulative Life Course Impairment), which characterizes the set of factors detrimental to the lives of patients with psoriasis resulting from stigma and physical and psychological disability, as well as different coping strategies.3 Furthermore, the inclusion of new biological drugs and small molecules, such as bimekizumab, brodalumab, spesolimab, tildrakizumab, and deucravacitinib. The new Consensus also highlights specific situations such as neoplasms, surgeries, and other procedures. It also innovates with the discussion of "Response Predictors" to contribute to and personalize psoriasis treatment and optimize results for each patient.
This Consensus maintained the Delphi methodology adopted in the 2020 consensus,1 which facilitates decision-making by seeking to standardize approaches to controversial issues in the literature.4,5 For this purpose, two rounds of questions were conducted, with anonymous participation from 80 and 76 authors, respectively, in each round. To ensure data validity, important criteria were adhered to, such as an adequate number of participants, representation from different regions of the country, the inclusion of experts from both the public and private healthcare systems, and the consistency of opposing questions in the same round. These measures help reduce potential biases. The consensus was considered reached when, for each question, at least 70% of voters assigned a score higher than 7 (Fig. 2).
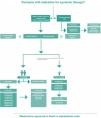
Finally, this Consensus updates the psoriasis treatment algorithm. For this purpose, the experts' responses were taken into consideration, and through the Delphi method, they reached an agreement on significant modifications compared to the 2020 Consensus. Among the new features of the updated severe psoriasis treatment algorithm, the authors highlight the removal of acitretin from the plaque psoriasis treatment sequence, with its use being reserved only for special situations. Deucravacinitib was also included in the treatment sequence, and treatment lines were defined for the selection of immunobiologicals. Anti-interleukin biologics were placed in the first-line position in relation to anti-TNF drugs. The treatment of psoriasis in the pediatric population was also included in the algorithm, with first- and second-line biologic treatments being defined. The algorithm (Fig. 3) includes a treatment sequence for flare-ups of generalized pustular psoriasis, with spesolimab and cyclosporine in the first-line therapy in this situation.
In addition to the recently approved targeted therapies described in the new Brazilian Psoriasis Consensus, new pathophysiological pathways have been targeted for blocking with biologics and small molecules, offering new future therapeutic perspectives.
Recently, pivotal studies involving the use of an oral peptide antagonist of interleukin receptors involved in the inflammatory cascade of psoriasis promise to offer another innovative, potentially effective, and safe treatment line for the management of this immune-mediated dermatosis. The outlook for interventions in psoriasis seems promising, the safety profile of new treatments has proven satisfactory, and the debate about a possible cure for psoriasis is more current than ever.6–8 Future updates to this consensus are therefore mandatory, allowing the discussion of increasingly effective and safe measures in the management of patients with psoriasis.
ORCID IDsRicardo Romiti: 0000-0003-0165-3831; André V.E. de Carvalho: 0000-0002-0407-538X; Juliana Nakano: 0000-0002-5638-3974
Authors' contributionsRicardo Romiti: Data curation; original draft; review and editing.
André V.E. de Carvalho: Data curation; original draft; review and editing.
Juliana Nakano: Data curation; original draft; review and editing.
Gleison V. Duarte: Data curation; original draft; review and editing.
Brazilian Society of Dermatology's Brazilian Psoriasis Consensus Working Group: Data curation; original draft.
FundingBrazilian Society of Dermatology.
Research data availabilityThe entire dataset supporting the results of this study was published in this article.
Ricardo Romiti: Consultant, research, and speaker activities for AbbVie, BMS, Boehringer-Ingelheim, Galderma, J&J, LeoPharma, Lilly, Novartis, Pfizer, Sanofi, SunPharma, and UCB.
André V. E. de Carvalho: Participated as a speaker, consultant, participant in medical events, or researcher with the following companies: AbbVie, BMS, Boehringer-Ingleheim, GSK, Johnson & Johnson, Leo Pharma, Lilly, Novartis, Sun Pharma, and UCB.
Juliana Nakano: Participated as a speaker, consultant, participant in medical events, or researcher with the following companies: AbbVie, BMS, Eli Lilly, Johnson & Johnson, Sanofi, and UCB.
Gleison V. Duarte: Participated as a speaker, consultant, participant in medical events or researcher with the following companies: Abbvie, BMS, GSK, Amgen, Boehringer-Ingleheim, Galderma, Johnson&Johnson, Leo Pharma, Lilly, Novartis, Sun Pharma, Celldex Therapeutics and UCB.
Adriana M. Porro
ORCID ID: 0000-0003-0736-4790
Affiliation: Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brazil.
Conflicts of interest: Abbvie, Janssen, Leofarma, Novartis, pfizer, boehringer, GSK.
Adriane Reichert Faria
ORCID ID: 0000-0002-1020-7211
Affiliation: Irmandade Santa Casa de Misericórdia de Curitiba, Curitiba, PR, Brazil.
Pontifícia Universidade Católica do Paraná, Curitiba, PR, Brazil
Conflicts of interest: None declared.
Aldejane Gurgel de A. Rodrigues
ORCID ID: 0009-0008-6394-5868
Affiliation: Universidade de Pernambuco, Recife, PE, Brazil
Conflicts of interest: Novartis, Janssen, UCB, Bristol, Abbvie
Alexandre Gripp
ORCID ID: 0000-0003-2217-6704
Affiliation: Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Conflicts of interest: None declared.
Aline Bressan
ORCID ID: 0000-0002-3296-5232
Affiliation: Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Conflicts of interest: None declared.
Aline Okita
ORCID ID: 0000-0002-3796-137X
Affiliation: Universidade de Mogi das Cruzes, Mogi das Cuzes, SP, Brazil.
Conflicts of interest: None declared.
Ana Carolina Belini Bazán Arruda
ORCID ID: 0000-0002-0371-2308
Affiliation: Pontifícia Universidade Católica de Campinas, Campinas, SP, Brazil
Conflicts of interest: SiAme Terapia Assistida, Novartis, Lilly, Abbvie, Johnson & Johnson, UCB, Leo Pharma.
Anber A. Tanaka
ORCID ID: 0000-0003-0963-0837
Affiliation: Faculdade Evangélica Mackenzie do Paraná, Curitiba, PR, Brazil
Conflicts of interest: Abbvie – conselho consultivo, consultor, palestrante, pesquisador; Boehringer-Ingelheim – conselho consultivo, consultor, palestrante, pesquisador; Eli-Lilly – conselho consultivo, consultor, palestrante, pesquisador; Janssen – conselho consultivo, consultor, palestrante; Leo Pharma – conselho consultivo, consultor, palestrante; Novartis – conselho consultivo, consultor, palestrante; pesquisador; UCB Biopharma – conselho consultivo, consultor, palestrante; Pfizer – palestrante, conselho consultivo; Sanofi – palestrante.
André Luís da Silva Hirayama
ORCID ID: 0000-0002-6276-3551
Affiliation: Faculty of Medicine, Hospital das Clínicas, Universidade de São Paulo, São Paulo, SP, Brazil
Conflicts of interest: Clinical research activities, consultant, lectures, support for participation in events Pfizer, Johnson & Johnson, AbbVie, Sun Pharma, Lilly, Novartis, Amgen, Sanofi, Leo Pharma, Boheringer.
André V.E. de Carvalho
ORCID ID: 0000-0002-0407-538X
Affiliation: Hospital Moinhos de Vento, Porto Alegre, RS, Brazil
Conflicts of interest: Has participated as a speaker, consultant, participant in medical events or researcher with the following companies: Abbvie, BMS, Boehringer-Ingleheim, GSK, Johnson&Johnson, Leo Pharma, Lilly, Novartis, Sun Pharma, UCB.
Andréa Machado C. Ramos
ORCID ID: 0000-0001-7414-3395
Affiliation: Service of Dermatology, Hospital das Clínicas, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil
Conflicts of interest: Janssen, UCB, Sanofi, Abbvie, Novartis.
Andréia Castanheiro da Costa
ORCID ID: 0000-0003-1732-3191
Affiliation: Centro Universitário Faculdade de Medicina do ABC, Santo André, SP, Brazil
Conflicts of interest: J&J, Abbivie, Novartis, UCB, Sunpharma.
Aripuanã Terena
ORCID ID: 0000-0002-2550-1341
Affiliation: Centro de Referência de Fototerapia e Fotobiologia Cutanea, Belo Horizonte, MG, Brazil
Conflicts of interest: Honorário da Abbvie, Johnson e Johnson, UCB Pharma, Lilly, Sanofi Novartis.
Arnóbio da Penha Pachêco
ORCID ID: 0000-0002-6904-7929
Affiliation: Hospital Universitário Onfre Lopes, Universidade Federal do Rio Grande do Norte, Natal, RN, Brazil.
Conflicts of interest: None declared.
Bethânia Cabral Cavalli Swiczar
ORCID ID: 0000-0003-2201-5628
Affiliation: IAMSPE and Complexo Hospitalar Padre Bento de Guarulhos, Guarulhos, SP, Brazil
Conflicts of interest: Speaker for Lilly, Janssen, Novartis, Boerringer, Leopharma, Abbvie
Bruna Elena Graciano Falcone
ORCID ID: 0000-0002-0467-6791
Affiliation: ABC Faculty of Medicine, São Paulo, SP, Brazil
Conflicts of interest: None declared.
Cacilda da Silva Souza
ORCID ID: 0000-0002-8157-7658
Affiliation: Department of Internal Medicine, Division of Dermatology, Ribeirão Preto Faculty of Medicine, Universidade de São Paulo, Ribeirão Preto, SP, Brazil
Conflicts of interest: Abbvie, BMS, Janssen, Leo-Pharma, Novartis, Sanofi, UCB.
Caio Cesar S. de Castroa,b
ORCID ID: 0000-0001-9767-5765
Affiliation:a Hospital de Dermatologia Sanitária do Paraná, Piraquara, PR, Brazil
b Pontifícia Universidade Católica do Paraná, Curitiba, PR, Brazil
Conflicts of interest: Abbvie, Lilly, Jansen, Sanofi and BMS- clinical research. Novartis – speaker.
Carlos Eduardo de Mathias Sanches
ORCID ID: 0000-0003-3459-2414
Affiliation: São José do Rio Preto Faculty of Medicine, São José do Rio Preto, SP, Brazil
Conflicts of interest: Novartis, Pfizer, Abbvie, Janssen, UCB, Leo Pharma, Lilly, Sun Pharma, Cerave, Sanofi.
Catarina Santos
ORCID ID: 0009-0005-0010-7777
Affiliation: Universidade Estadual de Ciências da Saúde de Alagoas, Maceió, AL, Brazil
Conflicts of interest: None declared.
Ciro Martins Gomes
ORCID ID: 0000-0002-3069-6884
Affiliation: Postgraduate Program in Medical Sciences, Faculty of Medicine, Universidade de Brasília, Brasília, DF, Brazil
Conflicts of interest: Speaker / Events: Abbvie, Novartis, BMS, Johnson & Johnson, UCB, Boehringer-Ingelheim.
Clarice Marie Kobata
ORCID ID: 0000-0001-9563-0707
Affiliation: Irmandade Santa Casa de Misericórdia de São Paulo, São Paulo, SP, Brazil
Conflicts of interest: Support for scientific events: Johnson & Johnson, Novartis, Bristol, UCB, Sunpharma, Lilly. Speaker : Johnson & Johnson, Novartis.
Cláudia Pires Amaral Maia
ORCID ID: 0000-0003-3264-1518
Affiliation: Policlinica Geral do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Conflicts of interest: Support in scientific events - ABBVIE, Janssen, Léo Pharma.
Claudio Lerer
ORCID ID: 0000-0002-0229-196X
Affiliation: Hospital Universitário de Brasília, Brasília, DF, Brazil
Conflicts of interest: AbbVie, Novartis, UCB, Johnson& Johnson, Sun Pharma.
Clivia Maria Oliveira
ORCID ID: 0000-0003-0406-360X
Affiliation: Universidade Federal do Pará, Belém, PA, Brazil
Conflicts of interest: Speaker: Abbvie, Johnson & Johnson, Galderma, Novartis, L'oreal, Sanofi, Boehringer, Leo Pharma, UCB. Advisory Board Galderma, Novartis, L'oreal, Sanofi, Johnson &Jonhson, UCB. Clinical research Eucerin, Novara. Scientific support: Jonhson & Johnson, Abbvie, Galderma, Novartis, L'oreal, Sanofi, Boehringer, Leo Pharma, UCB, Pfizer, Lilly.
Cynthia Cristina Ferreira Mota
ORCID ID: 0000-0002-3745-2559
Affiliation: Specialty Outpatient Clinic, Psoriasis Sector, Santos City Hall, Santos, SP, Brazil
Conflicts of interest: Jansen, Novartis, Abbvie and Sanofi, scientific support.
Daniel Holthausen Nunes
ORCID ID: 0000-0002-1303-5419
Affiliation: Universidade Federal de Santa Catarina, Florianópolis, SC, Brazil
Conflicts of interest: Speaker, Event Support, Advisory Board, Technical Material: AbbVie, Novartis, Janssen, UCB, Lilly, Sanofi, Leo Phrama, Boehringer.
Dimitri Luz
ORCID ID: 0000-0003-0869-9330
Affiliation: Universidade Santo Amaro, São Paulo, SP, Brazil
Conflicts of interest: Abbvie; Boehringer Ingelheim; Eli-Lilly; Janssen; Galderma; Leo Pharma; Novartis; Pfizer; Sanofi; Sunpharma; UCB Biopharma.
Domingos Jordão Neto
ORCID ID: 0000-0001-6244-7981
Affiliation: Hospital Heliópolis, São Paulo, SP, Brazil.
Conflicts of interest: Speaker Abbvie, Novartis and Janssen.
Eduardo Lacaz Martins
ORCID ID: 0000-0003-3563-9827
Affiliation: ABC Faculty of Medicine, São Paulo, SP, Brazil
Conflicts of interest: Jansen, Novartis, Lilly, UCB, Abbvie.
Esther Bastos Palitot
ORCID ID: 0000-0002-8195-2534
Affiliation: Centro de Pesquisa, Apoio e Tratamento de Psoriase da Paraíba, Universidade Federal da Paraíba, João Pessoa, PB, Brazil
Conflicts of interest: Speaker Novartis, Abbvie, Johnson & Johnson and UCB.
Fabrício Lamy
ORCID ID: 0009-0004-0214-5737
Affiliation: Policlínica Geral do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Conflicts of interest: Speaker and Member of the Advisory Board of Pharmaceutical Laboratories Johnson&Johnson, Lilly and Pfizer.
Francisca Regina O. Carneiro
ORCID ID: 0000-0001-6735-4004
Affiliation: Universidade do Estado do Pará, Belém, PA, Brazil
Conflicts of interest: None declared.
Gladys A. Martins
ORCID ID: 0000-0001-9913-2238
Affiliation: DermaCampbell, Brasília, DF, Brazil
Conflicts of interest: None declared.
Gleison V. Duarte
ORCID ID: 0000-0003-4160-5447
Affiliation: Dermatology Clinic, Instituto Bahiano de Imunoterapia, Salvador, BA, Brazil
Conflicts of interest: Has participated as a speaker, consultant, participant in medical events or researcher with the following companies: Abbvie, BMS, GSK, Amgen, Boehringer-Ingleheim, Galderma, Johnson&Johnson, Leo Pharma, Lilly, Novartis, Sun Pharma, Celldex Therapeutics and UCB.
Heitor de Sá Gonçalves
ORCID ID: 0000-0001-9062-2580
Affiliation: Centro de Referência Nacional em Dermatologia Sanitária Dona Libânia, Health Secretariat, Governo do Estado do Ceará, Fortaleza, CE, Brazil.
Conflicts of interest: None declared.
Jane Neffá
ORCID ID: 0000-0002-7888-0154
Affiliation: Hospital Universitário Antônio Pedro, Universidade Federal Fluminense, Niterói, RJ, Brazil
Conflicts of interest: None declared.
Jaquelini Barboza da Silva
ORCID ID: 0000-0002-5877-4290
Affiliation: Universidade de Santa Cruz do Sul, Santa Cruz do Sul, RS, Brazil
Conflicts of interest: Speaker Abbvie, Jannsen, Novartis, Sanofi.
João Carlos R. Avelleiraa,b
ORCID ID: 0000-0003-4683-7909
Affiliation:a Hospital Federal da Lagoa, Rio de Janeiro, RJ, Brazil
b Instituto de Dermatologia Prof.Azulay, Santa Casa de Misericórdia, Rio de Janeiro, RJ, Brazil
Conflicts of interest: None declared.
Juliana Catucci Bozaa,b
ORCID ID: 0000-0002-0573-1617
Affiliation:a Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil
b Hospital Moinhos de Vento, Porto Alegre, RS, Brazil
Conflicts of interest: Novartis, Abbvie, Johnson & Johnson, Lilly, Boehringer Ingelheim; La Roche Posay, Beiersdorf, UCB Biopharma, Sanofi, Vichy.
Juliana Nakanoa,b,c
ORCID ID: 0000-0002-5638-3974
Affiliation:a Irmandade Santa Casa de Misericórdia de São Paulo, São Paulo, SP, Brazil
b Hospital Israelita Albert Einstein, São Paulo, SP, Brazil
c Hospital Vila Nova Star, São Paulo, SP, Brazil
Conflicts of interest: Abbvie, Bristol Myers Squibb, Eli Lilly, J@J, Sanofi, UCB.
Juliana Viana Pinheiro Teixeira
ORCID ID: 0000-0002-8873-7095
Affiliation: Centro de Referência Nacional em Dermatologia Sanitária Dona Libânia, Health Secretariat, Governo do Estado do Ceará, Fortaleza, CE, Brazil
Conflicts of interest: None declared.
Juliana Yumi Massuda Serrano
ORCID ID: 0009-0000-7748-8583
Affiliation: Universidade Estadual de Campinas, Campinas, SP, Brazil
Conflicts of interest: Abbvie, Galderma, L´Oreal, Johnson & Johnson, Pfizer, Novartis, Sanofi, Takeda, UCB.
Leandro Linhares Leite
ORCID ID: 0000-0001-6370-3115
Affiliation: Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil
Conflicts of interest: Has served on the Advisory Boards of the pharmaceutical companies Janssen, Novartis, and Takeda. Has also served as a paid speaker for the pharmaceutical companies Novartis, Janssen, AbbVie, Lilly, BMS, UCB, Sanofi, and Boehringer. Has received support to participate in scientific events from the pharmaceutical companies Novartis, Janssen, Lilly, UCB, Sanofi, and Boehringer.
Leticia Oba Galvão
ORCID ID: 0000-0003-4486-1892
Affiliation: Hospital Regional da Asa Norte, Brasília, DF, Brazil
Conflicts of interest: Speaker: Johnson & Johson, Novartis, UCB, Abbvie, Pierre Fabre.
Ligia Pessoa de Meloa,b
ORCID ID: 0009-0006-5446-6860
Affiliation:a Hospital Otávio de Freitas, Recife, PE, Brazil
b Instituto de Medicina Integral Professor Fernando Figueira, Recife, PE, Brazil
Conflicts of interest: Classes: AbbVie, Eucerin, Janssen, Novartis, Lilly, Sanofi, UCB; Event support: Janssen; Lilly; Novartis; Sanofi; UCB. Promotional material preparation: Galderma; Sanofi.
Lincoln Fabricio
ORCID ID: 0000-0001-9191-1085
Affiliation: Faculdade Evangélica Mackenzie do Paraná, Curitiba, PR, Brazil
Conflicts of interest: Speaker, scientific support, consultant and clinical trial: Abbvie, Aché, Bayer, Bioderma, Galderma, Hypermarcas, Isdin, Johnson and Johnson, LaRoche-Posay, Leo-pharma, Novartis, Pfizer, Sanofi, Stieffel/Gsk and UCB.
Livia Barbosa
ORCID ID: 0000-0003-0326-1225
Affiliation: Private Prcatice Barbosa Souto Dermatologia, Rio de Janeiro, RJ, Brazil
Conflicts of interest: Jansen, Sanofi, Novartis, UCB, Abbive.
Lucas Campos Garcia
ORCID ID: 0000-0002-7883-5986
Affiliation: Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil
Conflicts of interest: None declared.
Luciane Donida Bartoldi Miot
ORCID ID: 0000-0002-2388-7842
Affiliation: Faculty of Medicine, Universidade Estadual Paulista, Botucatu, SP, Brazil
Conflicts of interest: Speaker Abbvie, Speaker Novartis, scientific research Janssen, scientific research Amgen.
Luciena C. Martins Ortigosa
ORCID ID: 0000-0002-3541-9456
Affiliation: Clínica Triune, Presidente Prudente, SP, Brazil
Conflicts of interest: None declared.
Luis Antonio de Paula Machado
ORCID ID: 0009-0007-0551-7286
Affiliation: Hospital BP Mirante, São Paulo, SP, Brazil
Conflicts of interest: Speaker Pfizer, Speaker Lilly, Speaker Leo-pharma publication Abbvie, publication Janssen.
Luíza Keiko Matsuka Oyafuso
ORCID ID: 0000-0003-3982-3322
Affiliation: ABC Faculty of Medicine, São Paulo, SP, Brazil
Conflicts of interest: None declared.
Luna Azulay-Abulafiaa,b
ORCID ID: 0000-0002-4698-2009
Affiliation:a Universidade do Estado Rio de Janeiro, Rio de Janeiro, RJ, Brazil
b Instituto de Dermatologia Professor Rubem David Azulay, Irmandade Santa Casa de Misericórdia do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Conflicts of interest: Researcher Lilly, Sanofi, Biogen, Astra Zeneca, Abbvie, Galderma, Janssen.
Marcelo Arnone
ORCID ID: 0000-0002-7192-5161
Affiliation: Department of Dermatology, Universidade de São Paulo, São Paulo, SP, Brazil
Conflicts of interest: Consultant and speaker: Abbvie, Bristol-Myers-Squibb, Janssen, Novartis, Pfizer, UCB-Biophama.
Marcelo Pinheiro
ORCID ID: 0000-0002-1896-8322
Affiliation: Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brazil
Conflicts of interest: Speaker J&J, UCB, Novartis, Lilly and Abbvie.
Marco Túlio Cavalcante Oliveira
ORCID ID: 0000-0002-9038-4911
Affiliation: Service of Dermatology, Hospital Universitário Walter Cantídio, Universidade Federal do Ceará, Fortaleza, CE, Brazil
Conflicts of interest: None declared.
Maria Cecilia de C. Bortoletto
ORCID ID: 0000-0002-6156-6097
Affiliation: Psoriasis Outpatient Clinic, Hospital do Servidor Público Municipal, São Paulo, SP, Brazil.
Conflicts of interest: None declared.
Maria de Fátima S. Paim de Oliveira
ORCID ID: 0000-0002-8594-200X
Affiliation: Universidade Federal da Bahia, Salvador, BA, Brazil
Conflicts of interest: Jansen, Novartis, Sanofi, Lily, UCB, Abbvie.
Maria Denise F. Takahashi
ORCID ID: 0000-0001-8671-6680
Affiliation: Faculty of Medicine, Universidade de São Paulo, São Paulo, SP, Brazil
Conflicts of interest: None declared.
María Victoria Suárez
ORCID ID: 0000-0002-2614-6011
Affiliation: Hospital do Servidor Público Municipal de São Paulo, Municipal Health Secretariat, São Paulo, SP, Brazil
Conflicts of interest: Researcher for Abbvie, Sanofi, Novartis, BMS. Speaker Novartis, J&J, Abbvie, Lilly, Sanofi, UCB, Leo Pharma.
Mauricio Amboni Conti
ORCID ID: 0000-0002-3502-1384
Affiliation: Hospital e Maternidade Marieta Konder Bornhausen, Itajaí, SC, Brazil.
Conflicts of interest: Speaker: Abbvie, Bristol, Johnson & Johnson, Lilly, Novartis, UCB. Participation in events: Abbvie, Bristol, Johnson & Johnson, Lilly, Novartis, Pfizer, Sun Pharma, UCB.
Mayra Ianhez
ORCID ID: 0000-0003-3604-3128
Affiliation: Hospital de Doenças Tropicais, Goiânia, GO, Brazil
Conflicts of interest: Abbvie, Sanofi, UCB, Bristol- Meyers- Squib, Galderma, Novartis, Janssen, Eucerin, FQM, Lilly, Pfizer.
Michelle dos Santos Diniz
ORCID ID: 0000-0002-9259-0807
Affiliation: Irmandade Santa Casa de Misericordia de Belo Horizonte, Belo Horizonte, MG, Brazil
Conflicts of interest: Abbvie, Novartis, Sanofi, Bristol, Lilly, Jansen, Leopharma, UCB, Pfizer.
Mônica Nunes de Souza Santos
ORCID ID: 0000-0003-0578-3270
Affiliation: Department of Dermatology, Faculty of Medicine, Universidade do Estado do Amazonas, Manaus, AM, Brazil
Conflicts of interest: None declared.
Paulo A. Oldani Felix
ORCID ID: 0000-0002-1055-0597
Affiliation: Hospital Federal dos Servidores do Estado, Rio de Janeiro, RJ, Brazil
Conflicts of interest: Speaker and consultant for Abbvie, Johnson & Johnson, Ucb, Bms, Sanofi, Leopharma, Eli Lilly, Galderma, Pfizer, Sandoz, Novartis.
Paulo Eduardo de Sá Gonçalves
ORCID ID: 0000-0001-7843-7497
Affiliation: Centro de Referência Nacional em Dermatologia Sanitária Dona Libânia, Health Secretariat, Governo do Estado do Ceará, Fortaleza, CE, Brazil
Conflicts of interest: None declared.
Ralph Vighi da Rosa
ORCID ID: 0000-0002-7608-3813
Affiliation: Department of Dermatology, Universidade Católica de Pelotas, Pelotas, RS, Brazil.
Conflicts of interest: Speaker Janssen e Novartis. Has received support for events from Janssen, Novartis, LeoPahrma and Abbvie.
Raquel Bissacotti Steglich
ORCID ID: 0000-0001-6550-3715
Affiliation: Universidade de Joinville, Joinville, SC, Brazil.
Conflicts of interest: Abbvie – speaker; Boehringer-Ingelheim – speaker; Janssen – speaker.
Renata Ferreira Magalhães
ORCID ID: 0000-0001-9170-932X
Affiliation: Department of Internal Medicine, Universidade Estadual de Campinas, Campinas, SP, Brazil.
Conflicts of interest: Abbvie, Johnson&Johnson, Novartis, lilly, Boehringer-Ingelheim, BMS, UCB, Pfizer, Leo Pharma, SunPharma, Sanofi.
Renata Rodrigues
ORCID ID: 0000-0002-0246-1316
Affiliation: Hospital Universitário Lauro Wanderley, Universidade Federal da Paraíba, João Pessoa, PB, Brazil
Conflicts of interest: Support for participation in scientific events: Sanofi, Abbvie, Pfizer, Novartis, Lilly, UCB, Molnlycke, Chiesi Farmacêutica, Smith and Nephew, Urgo; Advisory Board: Abbvie, Molnlycke; Preparation of scientific material: Sanofi, Molnlycke; Clinical research: Urgo; Speaker: Sanofi, Molnlycke.
Ricardo Romiti
ORCID ID: 0000-0003-0165-3831
Affiliation: Department of Dermatology, Hospital das Clínicas, Universidade de São Paulo, São Paulo, SP, Brazil.
Conflicts of interest: Consulting, research and speaker activities for Abbvie, BMS, Boehringer-Ingelheim, Galderma, J&J, LeoPharma, Lilly, Novartis, Pfizer, Sanofi, SunPharma, UCB.
Roberta Buense
ORCID ID: 0000-0003-4739-9699
Affiliation: Irmandade Santa Casa de Misericórdia de São Paulo, São Paulo, SP, Brazil.
Conflicts of interest: None declared.
Roberto Souto
ORCID ID: 0000-0001-7136-6008
Affiliation: Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
Conflicts of interest: None declared.
Rosana Lazzarini
ORCID ID: 0000-0002-4893-3593
Affiliation: Dermatology Clinic, Irmandade Santa Casa de Misericórdia de São Paulo, São Paulo, SP, Brazil
Conflicts of interest: Lilly Laboratories.
Samir de Figueiredo Azouz
ORCID ID: 0000-0002-8831-9926
Affiliation: Universidade Federal da Paraíba, João Pessoa, PB, Brazil.
Conflicts of interest: Abbvie, Sanofi, Novartis, Johnsson & Johnsson, Takeda, UCB, Bristol, Pfizer, Bristol, Lilly, MSD, Leo Pharma.
Sidney Augusto da C. Costa
ORCID ID: 0000-0002-5187-431X
Affiliation: Psoriasis Outpatient Clinic of the City Hall of Natal, Natal, RN, Brazil
Conflicts of interest: None declared.
Silvio Alencar Marques
ORCID ID: 0000-0002-5512-4700
Affiliation: Department of Infectious Diseases, Dermatology, Diagnostic Imaging and Radiotherapy, Faculty of Medicine, Universidade Estadual Paulista, Botucatu, SP, Brazil
Conflicts of interest: None declared.
Sineida Berbert Ferreira
ORCID ID: 0000-0001-7261-1220
Affiliation: Centro Paranaense de Estudos em Dermatologia, Maringá, PR, Brazil
Conflicts of interest: Speaker Lilly, Janssen, Novartis, UCB, Pfizer, Kenvue, Abbvie, Sanofi. Clinical research: Novartis, Sanofi, Ceped-UEM, Kenvue.
Sueli Carneiroa,b
ORCID ID: 0000-0001-7515-2365
Affiliation:a Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
b Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Conflicts of interest: Abbvie, Jansssen, Lilly, Novartis, Pfizer (speaker fees and support for Congresses and Courses).
Vanessa Lucilia Silveira de Medeiros
ORCID ID: 0000-0002-1445-587X
Affiliation: Universidade de Pernambuco, Recife, PE, Brazil.
Conflicts of interest: Johnson, Novartis, BMS, Abbvie, Boeringuer.
Verônica Rodrigues Bogado Leite
ORCID ID: 0000-0002-0232-2420
Affiliation: Department of Dermatology, Hospital Universitário Antônio Pedro, Universidade Federal Fluminense, Niterói, RJ, Brazil
Conflicts of interest: Janssen Novartis Sanofi UCB Abbvie Sun Pharma Bristol Lilly.
Vivianne Lira da C. Costa
ORCID ID: 0000-0002-2259-3236
Affiliation: Hospital Universitário Onofre Lopes, Universidade Federal do Rio Grande do Norte, Natal, RN, Brazil
Conflicts of interest: Novartis, Janssen, UCB, Lilly, Leopharma, Abbvie.
Wagner Galvão
ORCID ID: 0000-0001-6846-992X
Affiliation: Clínica ProDerma São Paulo, SP, Brazil
Conflicts of interest: Consulting activities and speaker for Abbvie, Boehringer-Ingelheim, J&J, LeoPharma, Lilly, Novartis, Pfizer, Sanofi, SunPharma, UCB.
Xinaida Taligare V. Lima
ORCID ID: 0000-0001-7999-6979
Affiliation: Universidade Federal do Ceará, Fortaleza, CE, Brazil
Conflicts of interest: Abbvie (Speaker), Amgen (Investigator), Biogen (Investigator), Johnson&Johnson (Investigator, Support for Scientific Events), Lilly (Speaker), Novartis (Speaker) and UCB Biopharma. (Speaker and Consultant).
The members of the Brazilian Consensus Working Group on Psoriasis of the Brazilian Society of Dermatology are listed in Appendix A.
Study conducted at the Department of Dermatology, Hospital das Clínicas, Universidade de São Paulo, São Paulo, SP, Brazil; Hospital Moinhos de Vento, Porto Alegre, RS, Brazil; Dermatology Clinic, Santa Casa de Misericórdia, São Paulo, SP, Brazil; Instituto Bahiano de Imunoterapia, Salvador, BA, Brazil.