Dear Editor,
Methotrexate (MTX) in low weekly doses is a first-line therapy for inflammatory diseases, such as moderate to severe psoriasis and rheumatoid arthritis1,2 due to its effectiveness, low cost and ease of use. Severe acute toxicity is rare and presents with mucositis, skin ulceration and pancytopenia.2 Factors such as age, drug interaction, individual susceptibility and comorbidities can contribute to the development of toxicity.3 However, the most common cause is a daily accidental ingestion, rather than weekly dose of methotrexate. The regular monitoring and the selection of patients for the use of this medication, appropriate counseling about drug interactions, adverse effects, as well as instructions on self-medication are essential to prevent complications. Misunderstanding about its use may lead to severe toxicity and even death.
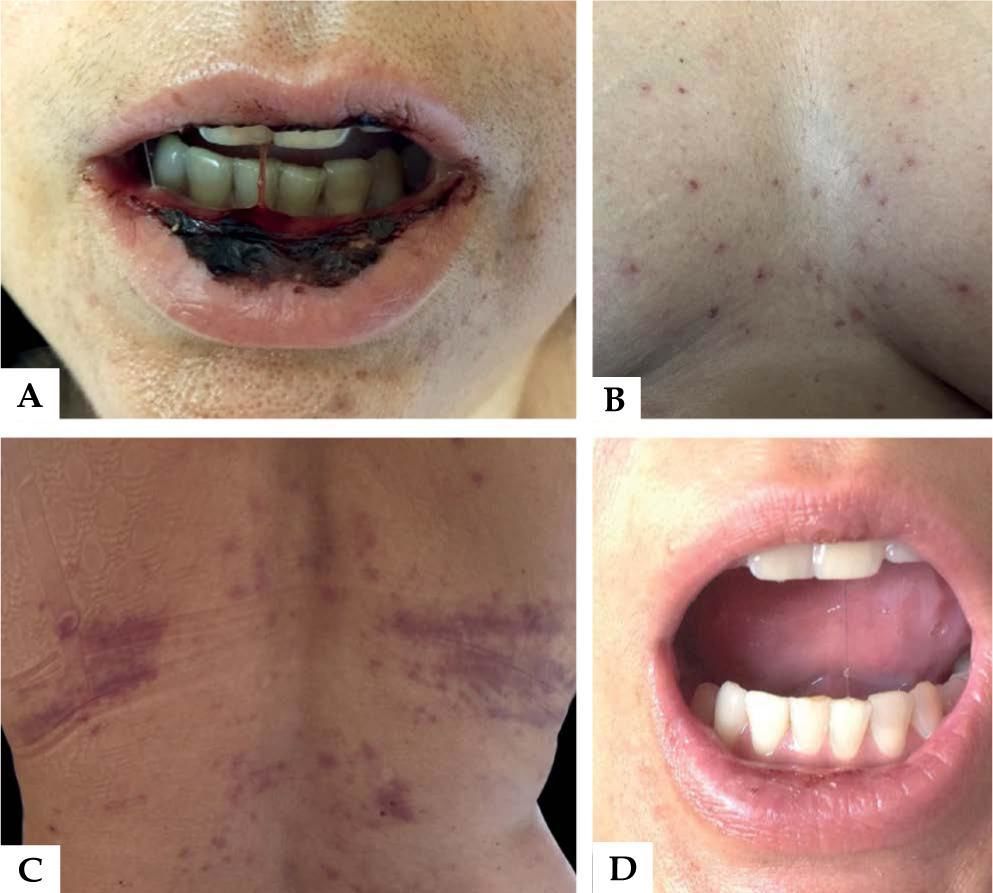
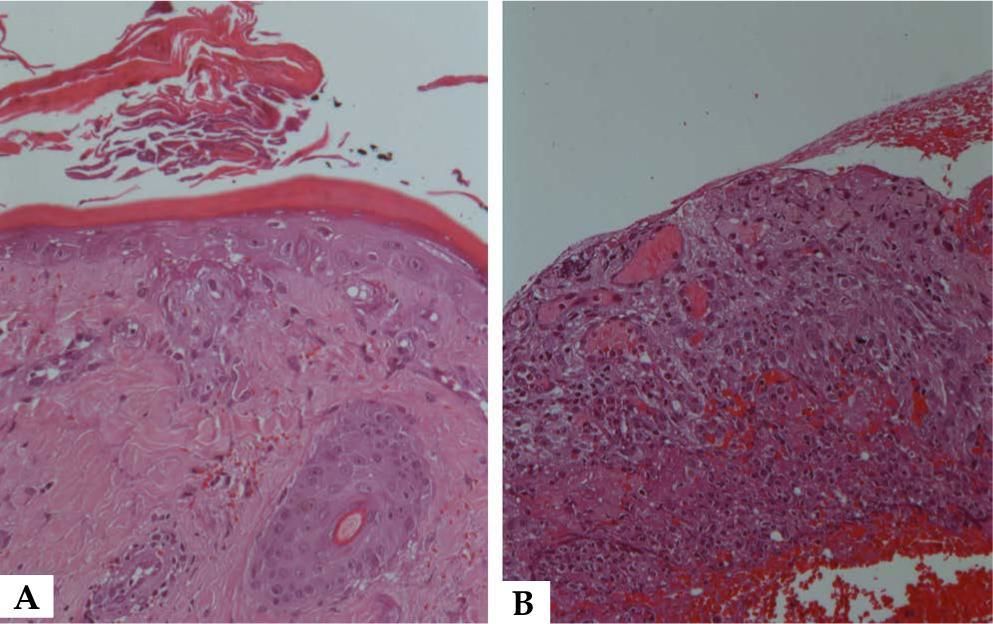
A 43-year-old woman recently diagnosed with rheumatoid arthritis has accidentally taken 15 mg of methotrexate daily, rather than weekly, for 9 consecutive days. She developed severe oral mucositis along with papules with central necrosis in the presternal region (Figure 1). Interestingly, areas of pressure were markedly involved (Figure 1). Laboratory tests showed pancytopenia (10.5 Hb, 1.940 leukocytes, 95.000 platelets) and hepatic dysfunction (AST 131 UI, ALT 200 IU). Light microscopy of the oral mucosa and the affected skin showed epidermal basal necrosis with discreet and superficial inflammatory infiltrate (Figure 2). The patient showed significant clinical and laboratory improvement after suspension of medication and replacement of folinic acid (Figure 1).
Methotrexate is generally administered once weekly to patients, with doses ranging from 7.5 to 25 mg/week. At the doses typically used, this medication has an anti-inflammatory effect as it increases the levels of intracellular adenosine. When used at high doses in oncology, it has anti-metabolic effects on cells with a high mitotic activity.2 Although safe and widely used in low doses, it is not free from side effects, which often lead patients to discontinuing the medication.4 The side effects of MTX are mainly gastrointestinal intolerance and hepatotoxicity. Although rare, some severe manifestations have been reported such as cytopenia, mucocutaneous toxicity, pneumonitis, neurotoxicity and nephropathy.5 The presence of oral mucositis, cutaneous ulcerations and pancytopenia (which may be followed by sepsis) suggest severe acute toxicity, since the drug inhibits rapid cell turnover.2
Pancytopenia presents within the first 10 days of treatment. It is dose dependent but occasionally it may be idiosyncratic. Mucositis usually occurs within the first 7 days of administration, prior to the onset of pancytopenia, as the accumulation of MTX is higher in mucosal epithelial cells than in the bone marrow stem cells. 2,4,5 Cutaneous involvement usually appears with mucositis. Its mechanism of action has been associated with direct drug toxicity in epithelial cells2, as seen in our patient. Histologically, this toxicity is evidenced by severe keratinocyte necrosis.1
The most common causes of acute MTX toxicity are dose errors and the concomitant use of medications, such as nonsteroidal anti-inflammatory drugs. Other factors, as renal function impairment, high alcohol intake, infections and advanced age may be involved, but overdose (daily dose instead of weekly dose) was the most common cause of acute MTX toxicity in reported cases.2 Prior to starting treatment with MTX, patients should be regularly monitored with renal, liver function tests, complete blood count. The patient’s age, the prevalence of comorbidities, the use of medications should be considered. It also requires knowledge of how often and how much of a medication should be given.1
MTX is an excellent therapeutic option for the treatment of inflammatory diseases, such as rheumatoid arthritis and psoriasis. However, due to its effectiveness and convenient posology, it can be indiscriminately used. Serious morbidity and potential mortality associated with acute toxicity justify the need for adequate guidance by the physicians and regular monitoring of patients receiving this type of therapy. It is of outmost importance that dermatologists are aware of a frequent misunderstanding about the dosage (daily instead of weekly dose).
Financial support: None.
Conflict of interest: None.