Orofacial granulomatosis is a nonspecific term that contains a wide variety of granulomatous entities, which share a clinical and histopathological presentation. It manifests as persistent or recurrent orofacial swelling, amongst other findings. Idiopathic orofacial granulomatosis, characterized by an absence of systemic granulomatous disease, is a diagnosis of exclusion. The main differential diagnosis is Crohn’s disease. Its pathogenesis is unknown, however, it seems to be immune-mediated. Patch-test sensitivity to multiple allergens is well documented. Currently, therapeutic options consider restrictive diets, topical, intralesional, and systemic agents. First-line therapy is currently a matter of debate. We present a review of the value of diet therapy in this syndrome, along with two illustrative cases.
Orofacial granulomatosis (OFG) is a nonspecific term encompassing a group of immunologically mediated, persistent or recurrent, inflammatory, granulomatous entities that manifest as soft tissue enlargement of the lips and face, with a spectrum of other orofacial features.1 It has been proposed that OFG with localized granulomatous inflammation of the lips (known as Miescher cheilitis), Crohn’s disease (CD) and Melkersson-Rosenthal syndrome (MRS) (lip edema, fissured tongue and facial paralysis triad) are all part of the same disease spectrum.1-3 There is no clear consensus on the definition of OFG, however, the main concern is to rule out CD.4
Treatment is often unsatisfactory.5 Numerous treatment strategies have been proposed, with no clear consensus on the matter. Pharmacological agents include topical (corticosteroids and calcineurin inhibitors), intralesional (corticosteroids), and systemic alternatives (corticosteroids, azathioprine, thalidomide, metronidazole and minocycline).1,6 At least three authors propose a cinnamon- and benzoate-free diet as first-line therapy.7-9 We present a review of the role of diet in the treatment of OFG, with two illustrating clinical cases treated with this same modality.
Report of the Cases&
Patient 1A 22-year-old Hispanic man presented to our oral medicine clinic with a one-year history of oral ulcers and a painless, intermittent, diffuse lower lip swelling. Over time, soft tissue enlargement became persistent.
He had a personal history of bronchial hyperresponsiveness and hay fever under treatment with oral fexofenadine. He denied any history of skin lesions, known allergies, food complaints or any symptoms of inflammatory bowel disease, present or past.
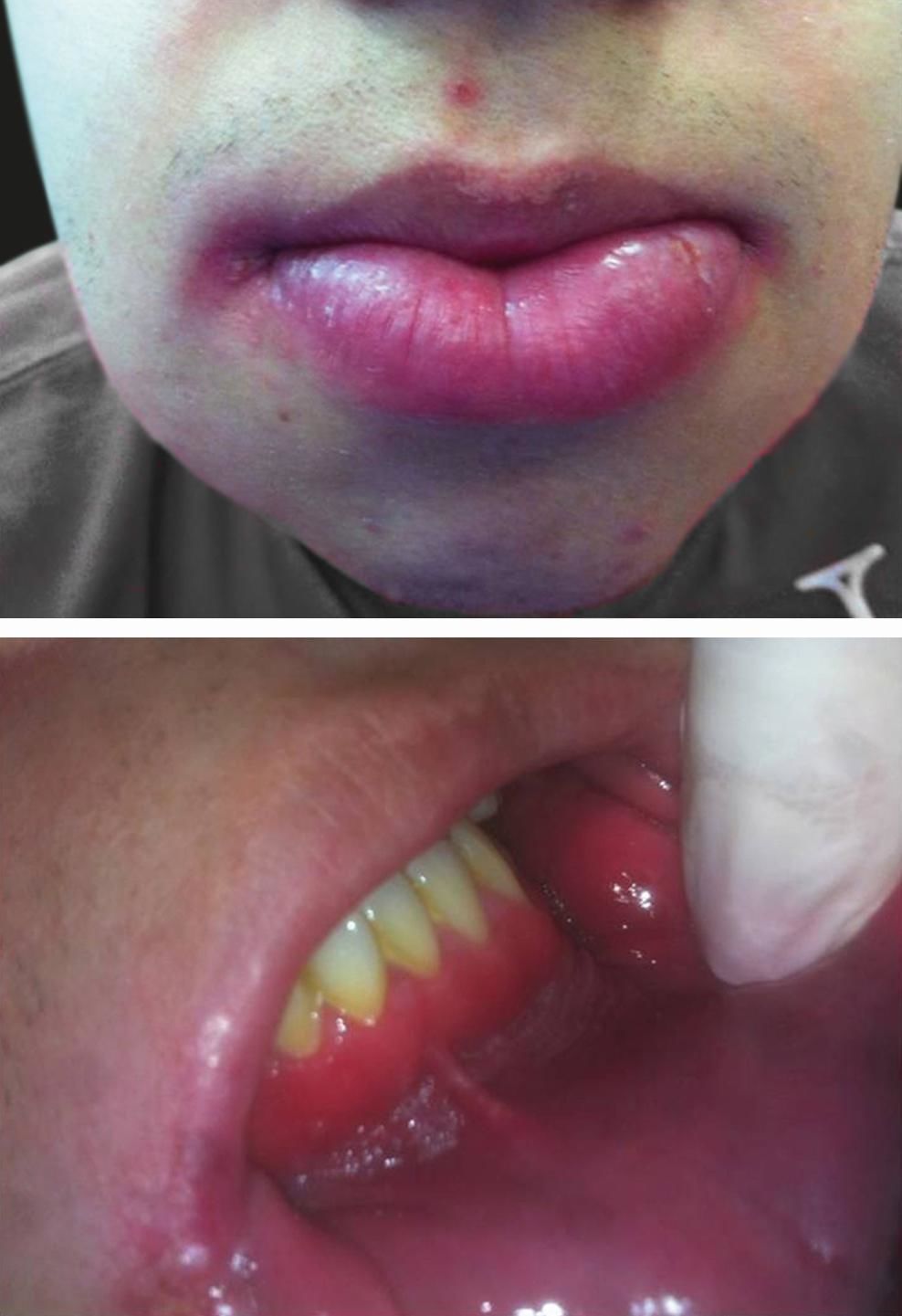
On physical examination, symmetrical lower lip swelling, cobblestoning of vestibular folds, “full width” gingivitis and angular cheilitis could be seen (Figure 1). There were no other relevant clinical findings.
After a clinical diagnosis of orofacial granulomatosis was made, a deep biopsy from the mucosa was taken. Histopathology showed a chronic inflammatory infiltrate, with noncaseating granulomas and giant multinucleate cells, histiocytes and lymphocytes (Figure 2).
With the diagnosis of orofacial granulomatosis, the patient was referred to a gastroenterologist to rule out Crohn’s disease and to an immunologist for the study of allergies.
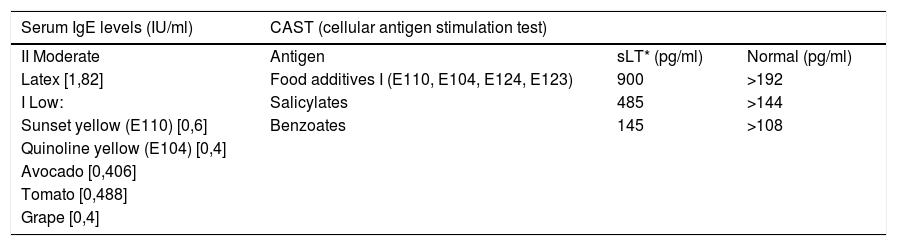
Specific serum IgE levels and a cellular antigen stimulation test (CAST) were performed, showing allergic hypersensitivity to certain food additives, latex, avocado, tomato and grapes (Table 1).
Specific serum IgE levels and cellular antigen stimulation test results from patient 1.
| Serum IgE levels (IU/ml) | CAST (cellular antigen stimulation test) | ||
|---|---|---|---|
| II Moderate | Antigen | sLT* (pg/ml) | Normal (pg/ml) |
| Latex [1,82] | Food additives I (E110, E104, E124, E123) | 900 | >192 |
| I Low: | Salicylates | 485 | >144 |
| Sunset yellow (E110) [0,6] | Benzoates | 145 | >108 |
| Quinoline yellow (E104) [0,4] | |||
| Avocado [0,406] | |||
| Tomato [0,488] | |||
| Grape [0,4] | |||
Computed tomographic enteroclysis showed no alterations in his digestive tract. Complete blood count, serum angiotensin converting enzyme levels, chest x-ray and CT scan, total IgG and IgM levels, c-ANCA, p-ANCA and atypical ANCA were all within normal range.
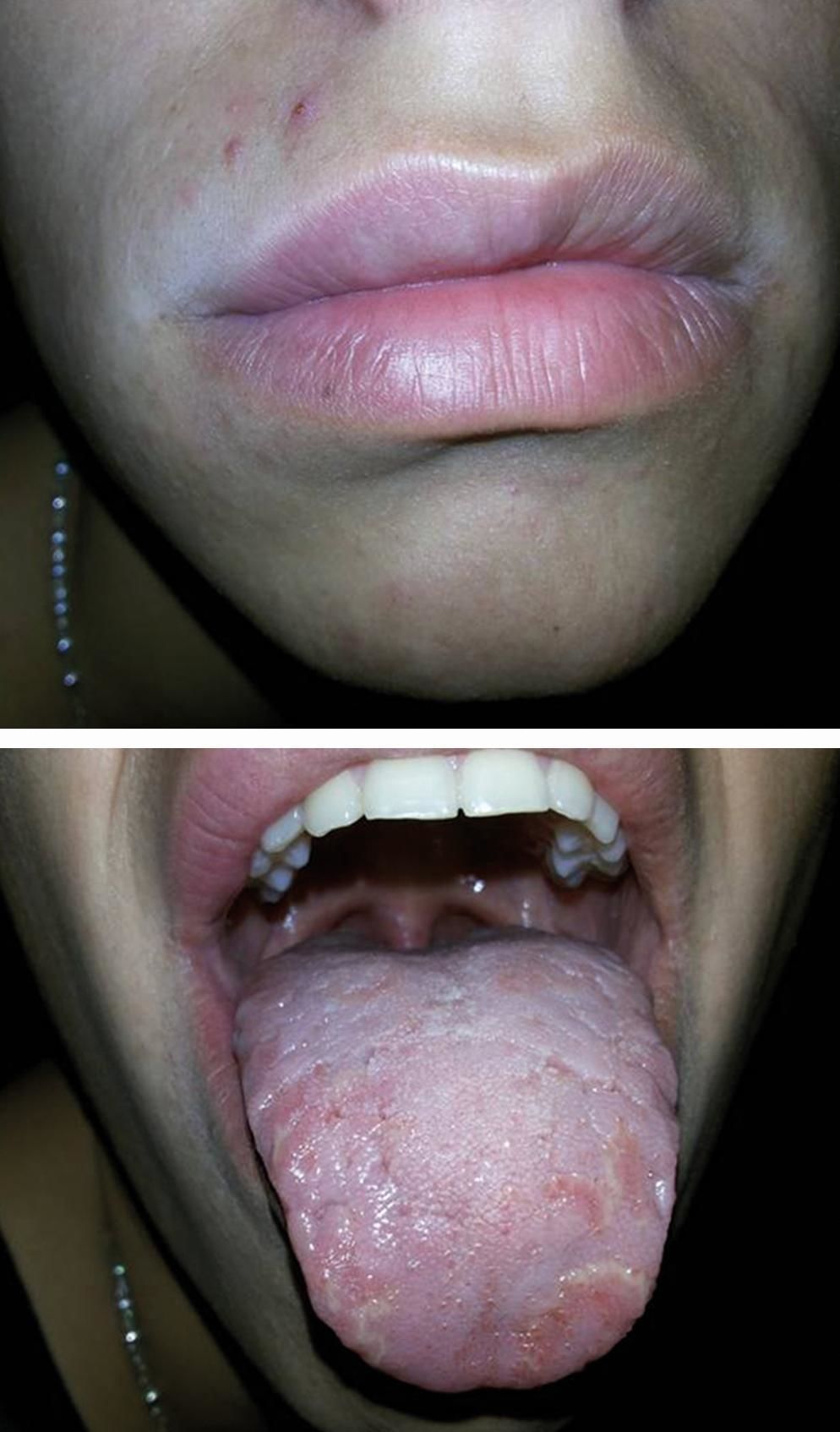
Our patient responded well to a cinnamon- and benzoate-free diet, avoiding additional allergens documented in his test results. He followed this diet for approximately three months, with notorious improvement in his lip swelling. He did not attend to regular follow-up controls. Eighteen months later, clinical evaluation showed improvement of the lip edema, but with slight cheek swelling, mainly towards his right side, with an asymmetric facial appearance (Figure 3). No mucosal alterations were present. He was quite satisfied with his current state, and was reluctant to continue with further treatments offered.
Patient 2A 15-year-old Hispanic man was referred to our oral medicine clinic with a 4-month history of a diffuse, painless and intermittent right upper lip swelling, as well as mild lower lip edema. He had no personal history of medication, skin lesions, known allergies, food complaints or symptoms of inflammatory bowel disease. He was referred by an immunologist with laboratory test showing normal levels of complement C3, C4 and C1 inhibitor, but total serum IgE levels were elevated.
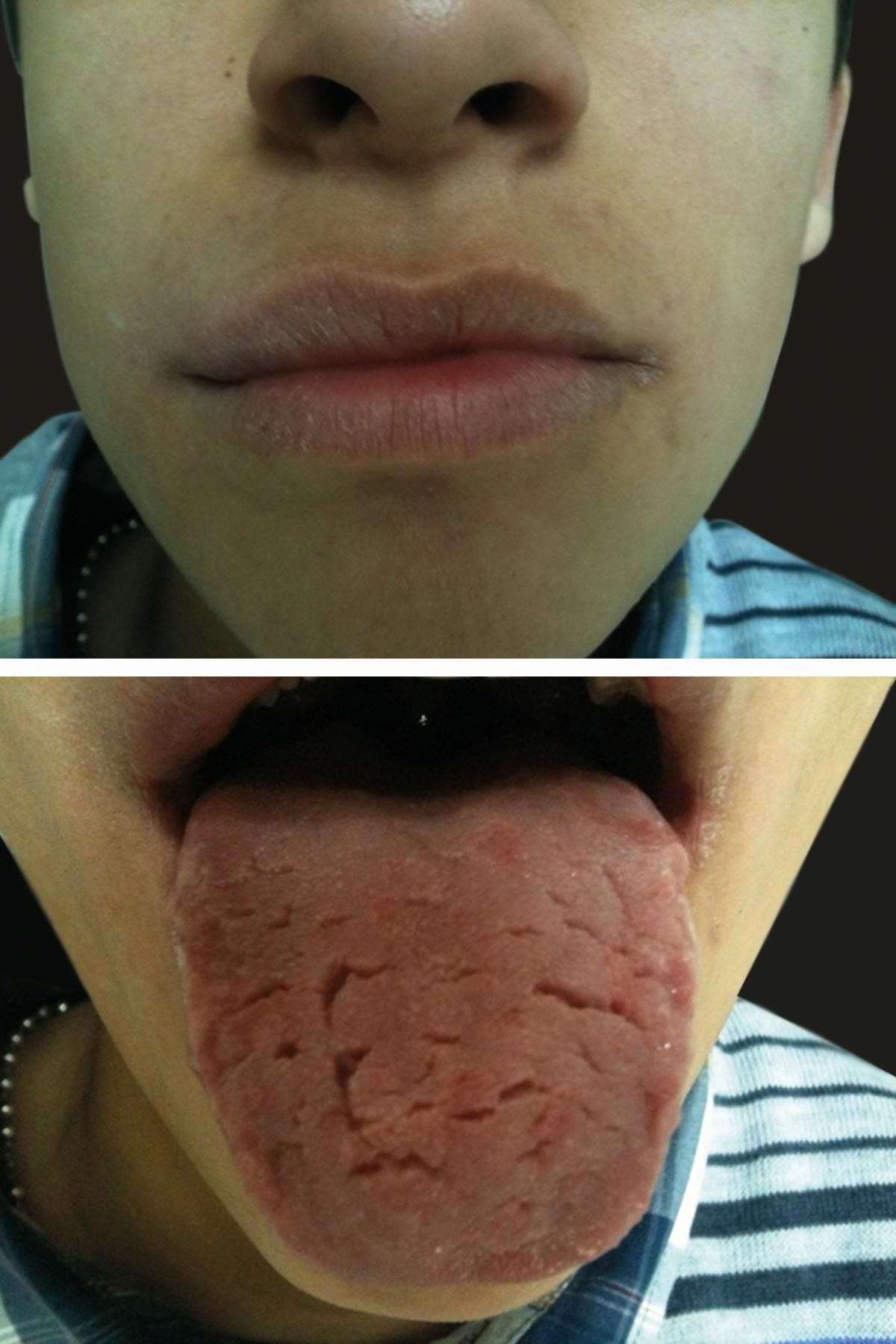
Physical examination revealed angular cheilitis and a fissured and geographic tongue. No neurological symptoms were evident (Figure 4).
With a clinical diagnosis of orofacial granulomatosis a deep biopsy from the upper lip was performed. Histopathology of lip tissue showed an inflammatory infiltrate with noncaseating granulomas and giant multinucleate cells. Minor salivary glands presented foci of lymphocytic infiltrate (not shown).
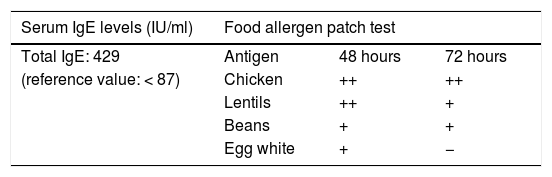
A food allergen patch test was performed, showing delayed hypersensitivity towards chicken, lentils, beans and egg whites (Table 2).
The patient responded well to a cinnamon- and benzoate-free diet, avoiding the additional allergens he tested positive for. He was assessed by a gastroenterologists who ruled out Crohn’s disease. During his follow-up, he presented no signs or symptoms of this disease. He continues to have a good clinical response to diet, with no systemic medication, with smaller orofacial swelling after two years of follow-up and a mild expression of geographic tongue (Figure 5).
DiscussionOFG is considered a rare entity, with unknown prevalence and no specific ethnic or gender predilection.5 Within the United Kingdom, the majority appears to be concentrated in Scotland.10 Though it may present at any age, it is more frequently diagnosed in children and young adults (median age of diagnosis: 28 years).11
The exact etiology of idiopathic OFG remains unknown. Current knowledge suggests a multifactorial relation between the immune system, environmental exposure and genetic susceptibility.10 Up to 80% have an IgE-mediated clinical allergy (e.g., hay fever, asthma, eczema and oral allergy syndrome). Its chronic nature points towards a delayed hypersensitivity reaction, nonetheless, patch testing tends to show immediate reactions more frequently.5,7 The association with atopy and B cells that express IgE in the lips suggests theses immunoglobulins may play a role.12
Idiopathic OFG has been linked to hypersensitivity reactions to multiple allergens, mainly detected through patch testing. A large review of 386 patients by Campbell et al.7 determined the most frequent food allergens that patch-tested positive in patients with OFG (Table 3). Patients may express subjective complaints towards certain foods, mainly chocolate, carbonated drinks, cinnamon and beer.11
Most common allergens detected through patch-testing in patients with OFG
| Allergen | Examples were they can be found | Percentage of patients that patch-tested positive (%) |
|---|---|---|
| Benzoic acid | Fruits and vegetables, especially berries and dried tomatoes | 36 |
| Food additives | 33 | |
| Perfumes and flavourings | 28 | |
| Cinnamaldehyde | Cinnamon, berries and hygiene products | 27 |
| Cinnamon | Food and hygiene products | 17 |
| Benzoates | Food additives E210-E219, soft drink preservatives, cinnamon, nutmeg, hygiene products and dental materials | 17 |
| Chocolate | 11 |
Wray et al. assessed 48 different allergens using cutaneous patch test in 264 patients with OFG.13 Sixty-two percent patch tested positive to one or more compounds; 51% were sensitive to benzoic acid and 38% to cinnamaldehyde. Contact urticaria (immediate response, type I hypersensitivity) to cinnamaldehyde and benzoic acid was common (36% and 46% respectively); however, delayed responses (type IV hypersensitivity) were infrequent: 7% and 4% for benzoic acid and cinnamaldehyde, respectively. Both reactions are detectable using patch test: type I during the first hours (e.g. 60 minutes), and type IV after 48 and 96 hours (standard patch testing). If immediate response is not assessed, and only standard patch testing is performed, there is a high risk of missing important allergens.14
Clinical manifestations vary during the course of the disease.5 OFG may show painless enlargement of the lips (upper and/or lower lip), fissuring, angular cheilitis, perioral erythema, swelling of the cheeks or cervical lymphadenopathies. Oral examination may reveal cobblestoning, ulceration (which may be painful), gingivitis, tags and fissured tongue.11 Lip edema is the most common finding (over 90%), although it is rarely the sole clinical feature.5 Swelling usually lasts from hours to weeks, but with time both frequency and duration increase until swelling becomes persistent and firm.1,5,15 In a minority of cases, manifestations can affect other parts of the face.
The prevalence of facial palsy is unclear (8% - 57%).5 Other neurologic symptoms may exist.3,16
Histopathology should always reveal a chronic inflammatory infiltrate.17 Although it is considered a granulomatous entity, less than half of patients have noncaseating granulomas, usually small and poorly defined, with lymphocytes surrounding epithelioid histiocytes1,17 Multinucleated giant cells can also be seen, with edema of the corium, lymphangiectasia and perivascular lymphocytic infiltration.11
Laboratory findings may show anemia, lymphocytopenia, low ferritin, with elevated levels of C-reactive protein, IgE levels and alkaline phosphatase.11 Though these values may be altered in idiopathic OFG, they should raise suspicion about a possible underlying CD. Gastrointestinal symptoms should also alert the physician, as well as oral ulcerations, oral scarring and sulcal involvement.
Differential diagnosis includes CD, mycobacterial infections, sarcoidosis and foreign body reactions.18 Both physical examination and biopsy of oral lesions are can not distinguish OFG, MRS or CD.2,4,11,19
Idiopathic OFG is considered a diagnosis of exclusion.18 A thorough clinical examination of digestive and extra-digestive signs of CD must be performed. Gastroenterology evaluation is advisable, and sometimes endoscopy with biopsy is considered necessary. Some also recommend determining serum angiotensin converting enzyme levels, a tuberculin skin test, chest radiography, Ziehl-Neelsen stain and polarized light microscopy in histopathology.1
This condition may affect quality of life due to aesthetic concerns, painful oral ulcerations and occasional neurologic symptoms. A multidisciplinary approach is needed, involving oral pathologists, dermatologist, gastroenterologist, nutritionist, clinical immunology/allergy specialists, and sometimes a mental health specialist. If signs and symptoms are mild, treatment may not be necessary.5
First-line therapy is currently a matter of debate, with no single therapeutic alternative showing consistent efficacy or predictability.5
Some authors consider restrictive diets as first-line treatment, with good response and better acceptability to a cinnamon- and benzoate-free diet over pharmacological agents.7,8,9 This is based in the fact that cinnamon and benzoate compounds are referred to as the most common triggers.5 Chocolate also merits avoidance. Properly supervised, it is nutritionally adequate and, according to a review by Campbell et al., it was deemed beneficial in 54% - 78% of patients, with 23% requiring no adjunctive therapies.7 Patch testing does not predict dietary outcome (40% of patients who tested negative still responded to the diet). This diet should be strict and undertaken for at least 12 weeks, with follow-up every 4 - 6 weeks. Clinical improvement is generally seen within the first few weeks. If there are no significant changes after 12 weeks, medical alternatives can be added and other allergens should be considered. Dietary re-introduction may take place eventually, with the appropriate supervision. A dietary tool guideline is available in www.kcl.ac.uk/ofg.7
The cinnamon- and benzoate-free diet does not completely exclude phenolic acid. Both benzoic acid and cinnamon are considered to be sources of this compound. Campbell et al.20 designed a prospective, open-label, intervention study of dietary exclusion and controlled reintroduction of phenolic acids in patients with OFG. Seven out of 10 patients showed improvement in their symptoms, and 4 out of 6 patients that had not responded to a standard cinnamon- and benzoate-free diet had positive results to this low phenolic acid diet. A phenolic acid-free diet is extremely restrictive, requiring micronutrient supplementation. This renders it impractical, and since clinical results are modest, it is not advisable as a routine therapeutic option.
Campbell et al.12 performed a study in which patients diagnosed with OFG and with a positive skin prick test to silver birch, grass, mugwort, ragweed and latex underwent a dietary program avoiding cross-reacting foods to these allergens. This diet was carried out for 6 weeks and, in those who responded, for 12 weeks in total. Only 2 out of 14 patients (14.3%) showed significant improvement and did not require additional treatment. The small number of patients in this study was a limitation, and poor response determines that this diet might not be recommended in mainstream clinical practice.
Strict dietary adherence is extremely difficult to achieve, and response to diet restriction is variable among existing trials, with little replicability.15 Methodological limitations include concomitant use of other treatment agents in the experimental population, lack of control groups and open-label designs.9,12 The fact that patch testing has such a low correlation with results must be considered and requires further insight. Currently, we believe it is necessary more evidence to support diet as a therapeutic option by itself, although it may be very useful as an adjuvant therapy when compliance is possible.
Recent trials have shown that intralesional corticosteroid injections (triamcinolone acetonide 40 mg/ml) in mild and moderate cases of idiopathic OFG. In a clinical trial with 22 patients by Fedele et al., 63.6% of patients had significant improvement of swelling, with no recurrence of OFG for up to 4 years after a single course of therapy. The ones who showed a reappearance of orofacial edema were treated successfully with a second course of intralesional triamcinolone. All patients in this trial had no swelling after four weeks, and most (20/22; 91%) had achieved this within the first two weeks.5,21
In acute swelling, cold compresses, lip lubricants and emollients can be used.3 Ulceration of the oral mucosa, mucosal tags, and cobblestoning are usually managed with topical corticosteroids and are rarely severe enough to require systemic agents. Mild orofacial swelling can be treated with topical corticosteroids, calcineurin inhibitors or both. Oral candidiasis is the most frequent adverse event of topical therapy.1
Systemic corticosteroids usually have short-lived benefits, and chronic use is associated with undesirable adverse events. Other systemic immunosuppressants are probably a safer option in the long-term, mainly mycophenolate mofetil, azathioprine, thalidomide, infliximab and adalimumab.5
Surgery should only be considered in severely disfiguring cases, after medical treatment has proven insufficient, preferably during a quiescent phase of the disease.19
Follow-up is important, since treatment is usually programed in a long-term basis. Prognosis is good, but patients must be informed that recurrence is common and often unexplained.7 Clinical outcome is unpredictable, however, persistent orofacial swelling can be prevented when OFG is diagnosed and treated early on.17,19 Neurologic signs usually appear at an initial stage and are unlikely to develop afterwards.1 Long-term follow-up is particularly important in patients under 16-years-old, since they seem to have a higher risk of developing CD later in life (up to 40%).2,4,22
OFG represents a diagnostic challenge, considering clinical onset can be highly polymorphous, and, due to its rarity, it is not very well known in the medical community.16 There is still no consensus on the definition of OFG, and even though certain treatment alternatives have proven beneficial, more information is needed before a definite recommendation can be formulated.
One of the main difficulties in establishing a therapeutic course of action relates to the fact that this disease has a very low prevalence, and gathering enough cases to perform a study with statistically significant results remains a challenge. Future investigations in dietary therapy should consider a multicenter approach, adding control groups and performing double blind randomized controlled trials.
In both our cases we were able to see a good initial response to restriction of the allergens that they tested positive for; however, long-term results were partial but acceptable by the patients. Due to the lower invasiveness, we recommend diet control as the first-line therapy, even if this is not the definitive treatment and requires adjunctive therapies.
AcknowledgementWe are very grateful to Dr. María Elena McNab for her help in preparing this manuscript.
Financial support: none.
Conflict of interest: none.