Livedoid vasculopathy (LV) is a rare, chronic, recurrent vascular disease that manifests as erythema, purpuric macules, painful ulcers, livedo reticularis, atrophic porcelain-white scars of the lower extremities, typically in ankles and feet. Although the pathophysiological process is not yet fully elucidated, thrombosis of the dermal vessels appears to be a key event.1 The conventional therapies consist of anticoagulants, antiplatelets, corticosteroids, immunosuppressive agents, thrombolytics,2 etc. However, patients commonly encounter disease relapse. Upadacitinib, a relatively new JAK1 inhibitor, has never been reported to be used in treating LV in the retrieved literature. Herein, we report a case of refractory LV resistant to conventional therapy which exhibited great efficacy following treatment with Upadacitinib.
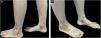
A 30-year-old female presented with over 5-year history of painful, ulcerative lesions of the feet and ankles. Physical examination showed multiple purpura maculae, painful ulcerations with small, dilated blood vessels, as well as sporadic porcelain-white scars (Fig. 1). Laboratory investigations were found with positive anti-cardiolipin antibody, and helped us rule out other LV-associated systemic disorders. A skin biopsy revealed ulceration formation, vascular dilation, swollen vessel walls with fibrinoid necrosis, erythrocyte exosmosis, as well as infiltration of perivascular lymphocytes and neutrophils (Fig. 2). The examination findings and clinical manifestations were strongly consistent with the diagnosis of LV. Although the patient was previously prescribed rivaroxaban, aspirin, mycophenolate morphenate, and oral corticosteroids (prednisone 30 mg once daily), she was still confronted with disease relapse. We initiated Upadacitinib at a daily dose of 15 mg orally, along with topical ointment, hirudoid (mucopolysaccharide polysulfate cream). The patient manifested significant improvement in erythema and pain within 10-days, and achieved nearly complete resolution of ulceration and remained only post-inflammatory hyperpigmentation within 6-weeks (Fig. 3). No adverse events were observed during the 20-week follow-up. For clinical assessment, the expected time for Upadacitinib maintenance is 6-months, so as to reduce the recurrence of the patient.
Pathological and immunohistochemical specimens. (A) Low-power view showed ulceration formation, increased vascular proliferation and dilation (Hematoxylin & eosin, ×50). (B) The infiltration of perivascular lymphocytes and swollen vessel walls with fibrinoid necrosis, erythrocyte exosmosis (original magnification: ×400).
The Janus Kinase (JAK) and Signal Transduction Transcription Activator (STAT) signal transduction pathways interplay with a wide range of immunocytes and over 50 cytokines. It was reported JAK-STAT pathways have enhanced activity in vascular endothelial cells;3 moreover, JAK signaling could significantly affect inflammation, coagulation, and thrombosis formation.4 The overactivation of JAK/STAT pathways induces immune imbalance and vascular damage.
LV is a thrombo-occlusive vascular disorder, which on histopathology manifests as hyaline thrombosis, fibrin deposits in vessel walls, along with perivascular focal lymphocyte infiltration. As described previously, it is still difficult to treat and prone to relapse. Existing experience suggests that steroids were suggested to be effective even as a monotherapy, and the traditional anti-inflammatory drugs could successfully improve the clinical outcomes. Concurrently, employing colchicine and prednisolone as monotherapy could exhibit higher efficacy than using pentoxifylline and aspirin alone in LV patients, indicating an indispensable role of inflammation in LV pathogenesis.5 Given its pathophysiology, JAK inhibitors should theoretically play an important role in preventing the flares and recurrence of LV.
Previous small-scale retrospective studies and case reports have shown considerable efficacy when utilizing JAK inhibitors in LV treatment,5,6 as summarized in Table 1. Han et al. observed 8 patients who received 2 mg/day of baricitinib for treating refractory LV, and found that all enrolled patients experienced a significant regression with a mean remission time of 7.75-weeks. Similarly, in the case series of Song et al., 3 patients with LV were prescribed 2 mg/day of baricitinib. One patient obtained darkening and reduced erythema by month-6, and the other two obtained complete ulcer healing, respectively, by month-1 and month-2. Regarding other JAK inhibitors, Chen et al. reported the rapid improvement in a 31-year-old female developing LV after using abrocitinib 100 mg once daily. In addition, Jia et al. administered tofacitinib 5 mg twice per day on a 17-year-old male with refractory LV, the ulcers were completely healed within a month with no recurrence during the 56-week follow-up. The aforementioned clinical practice suggests that JAK inhibitors are promising therapeutic options to treat LV. Upadacitinib is an oral, small-molecule JAK1 inhibitor that has been approved for multiple inflammatory diseases in dermatology. There are no reports to date for its application on LV. In our case, using 15 mg/day of Upadacitinib, the patient achieved great improvement within 10-days and the ulceration completely healed within 6-weeks.
Overview of JAK inhibitors as a novel treatment for livedoid vasculopathy.
| Study | No. | Sex | Age | Duration of Disease | Previous Treatment | Treatment | Outcome | Adverse event | Follow-up time |
|---|---|---|---|---|---|---|---|---|---|
| Han et al. | 8 | 5F/3M | 8∼36 | 7∼120 months | Diverse therapies (Aspirin, corticosteroid; thalidomide; tripterygium glycosides; rivaroxaban; enoxaparin; compound glycyrrhizin; Chinese traditional anti-inflammatory drugs) | Baricitinib 2 mg/day | All experienced significant regression; remission times ranging from 3- to 13-weeks, with a mean remission time of 7.75 ± 3.45 weeks | None | 11‒28 weeks |
| Chen et al. | 1 | 1F | 31 | 2-years | NR | Abrocitinib 100 mg/day | Complete remission was achieved after 6-weeks, with only post-inflammatory hyperpigmentation remaining | None | 12-weeks |
| Song et al. | 3 | 1F/2M | 8‒26 | 6∼72 months | Diverse therapies (Prednisone, rivaroxaban, thalidomide, aspirin) | Baricitinib 2 mg/day | Patient 1: reduced erythema by month-6; Patient 2/3: Complete healing of ulcer by month 1/2 | None | |
| Jia et al. | 1 | 1M | 17 | 3-years | Colchicine, thalidomide, dipyridamole, rivaroxaban and aspirin | Tofacitinib 5 mg twice daily | Complete healing of ulcer by month-1 | None | 56-weeks |
JAK, Janus Kinase; M, Male; F, Female; NR, Not Report.
To our knowledge, this is the first report in which Upadacitinib was utilized to treat LV. Further studies should be conducted to confirm its efficacy and long-term safety.
ORCID IDsXiaoqian Liang: 0000-0001-8806-8533; Xueyi Huang: 0000-0002-3114-589X
Authors’ contributionsJiecheng Zheng: The study concept and design; data collection, or analysis and interpretation of data; Writing of the manuscript or critical review of important intellectual content; Data collection, analysis and interpretation.
Xiaoqian Liang: The study concept and design; data collection, or analysis and interpretation of data; Writing of the manuscript or critical review of important intellectual content; Data collection, analysis and interpretation.
Xueyi Huang: Data collection, or analysis and interpretation of data.
Jia Liao: Effective participation in the research guidance; Final approval of the final version of the manuscript.
Financial supportThis research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Research data availabilityDoes not apply.
None declared.