Background: Renal transplant recipients are submitted to immunosuppression to avoid graft rejection, which makes them susceptible to various conditions. Furthermore, these individuals present malignant tumors more frequently than the general population, including nonmelanoma skin cancer. The individual genetic basis that acts in the pathogenesis of cutaneous cancer may present a protection or susceptibility factor for disease development. One of these factors is the HLA complex.
Objective: To investigate HLA alleles association to the occurrence of nonmelanoma skin cancer in renal transplant recipients from Sao Paulo State.
Methods: A total of 213 patients (93 renal transplant recipients with nonmelanoma skin cancer and 120 renal transplant recipients without nonmelanoma skin cancer) were evaluated by retrospective and cross-sectional study. Epidemiological, clinical and HLA typing data were found in databases. HLA class I (A, B) and class II (DR) alleles were compared to establish their association with nonmelanoma skin cancer.
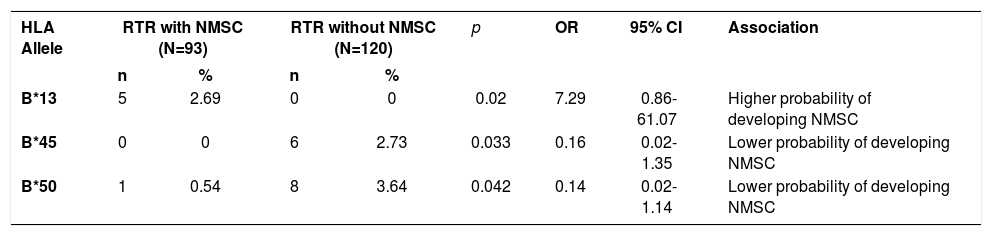
Results: Comparing renal transplant recipients with and without nonmelanoma skin cancer, the HLA-B*13 allele was associated with higher risk of developing nonmelanoma skin cancer while B*45 and B*50 alleles were associated with protection.
Study limitations: The HLA A, B and DR alleles identification for the kidney transplantation routine is done by low and medium resolution techniques that do not allow discrimination of specific alleles.
Conclusion: The involvement of HLA alleles in nonmelanoma skin cancer in renal transplant recipients was confirmed in this study. Renal transplant recipients with HLA-B*13 showed higher risk for developing a skin cancer (OR= 7.29) and should be monitored for a long period of time after transplantation.
Renal transplant recipients (RTR) are subject to iatrogenic immunosuppression to prevent graft rejection, what makes them susceptible to many diseases. Furthermore, these individuals develop malignant tumors more frequently when compared to the general population, including nonmelanoma skin cancers (NMSC).1–6 Multiple factors work together in promoting an increased risk for the development of skin cancer, among them the individual genetic basis, that can confer protection or susceptibility for the development of the condition. HLA complex (Human Leukocyte Antigen) is one of those determinants.7,8
The detection of susceptibility or protection alleles in RTRs allows the characterization of the risk profile for the development of NMSC. The identification of high risk patients will enable more effective preventative actions, such as change in habits (use of sunscreen and of sun protective clothing), routine dermatological consultations with the treatment of pre-malignant lesions and change of immunosuppression to mTOR inhibitors (mammalian target of rapamycin).
MethodsPatients: a retrospective, cross-sectional study was conducted at the Department of Dermatology of the Universidade Federal de Sao Paulo - Escola Paulista de Medicina (Unifesp-EPM), Hospital Sao Paulo and Hospital do Rim e Hipertensao, from July 2004 to July 2014.
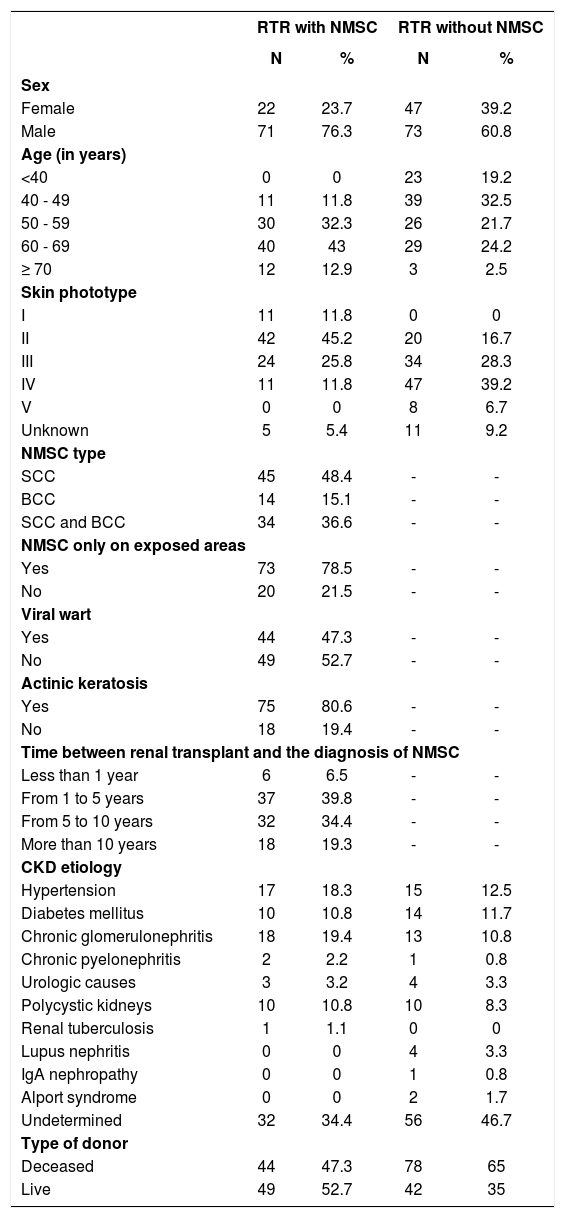
A total of 213 RTRs (93 with NMSC and 120 without) were included. Epidemiological, clinical and laboratory data of the RTRs were obtained from electronic medical charts from Hospital Sao Paulo and the Department of Dermatology, Unifesp-EPM.
The following clinical variables were studied: sex, age, phototype, etiology of chronic kidney disease, type of organ donor (live or deceased), type of NMSC (basal cell or squamous cell carcinoma), NMSC site, presence of viral warts and actinic keratosis and interval between the transplant and he diagnosis of NMSC.
HLA typing: HLA class I (A* and B*) and class II (DRB1*) were genotyped by PCR-SSP (polymerase chain reaction with sequence-specific primers), with the commercial kit Micro SSPTM (One Lambda®, CA, USA) and/or PCR-SSO (polymerase chain reaction sequence-specific oligonucleotide) with the technique LabtypeTM (One Lambda®, Canoga Park, CA, USA). HLA typing is routinely performed for solid organ transplant. These data were collected from the Hospital do Rim e Hipertensao database.
Statistical analysis: allele frequency was determined by direct count. Hardy-Weinberg principle was tested to confirm the frequency distribution of alleles using the software Arlequin, version 3.0. Comparative analysis of HLA alleles from RTRs with and without NMSC was performed with the Chi-square test or Fisher’s exact tests with Graph Pad (http://www.graphpad.com/quickcalcs/contingencyl.cfm). Values of p lower or equal to 0.05 were considered significant. The odds ratio was calculated with a confidence interval of 95% (95%CI), using SISA (http://www.quantitativeskills.com/sisa/) when p values were significant.
Ethical aspects: this project was approved by Unifesp Committee of Ethics in Research (under the number 758.199 on 08/20/2014).
ResultsAmong RTRs with NMSC, 71 patients were male (76.3%). Mean age was 60 years, ranging from 40 to 83 years. The patient’s skin phototype was classified according to the Fitzpatrick scale, ranging from I and IV. The predominant skin type in this population was II (45.2%), followed by type III (25.8%). Phototype I had a prevalence of 11.8% as did type IV.
Regarding renal transplant, the etiology of chronic kidney disease (CKD) for the majority of RTRs with NMSC was undetermined (34.4%). Among the defined etiologies for CKD, 19.4% had chronic glomerulonephritis; 18.3%, hypertension; 10.8%, diabetes mellitus; 10.8%, polycystic kidney disease; 3.2%, urologic etiology; 2.2%, chronic pyelonephritis; and 1.1%, renal tuberculosis. Renal transplant was from a live donor in 52.7% of patients. For post-transplant immunosuppression, most patients used three medications, while only two patients used two. The medications most commonly used were: prednisone, azathioprine, cyclosporin A and tacrolimus.
In the analysis of NMSC parameters, considering the total number of cases presented, we found a higher prevalence of squamous cell carcinoma (SCC) (84.9%) and 36.6% had SCC and basal cell carcinoma (BCC). BCC appeared on its own in 15.1% of the sample. NMSC was diagnosed exclusively in areas exposed to sun light (face, scalp, neck and lower limbs) in 78.5% of RTRs. The diagnosis of viral warts was made in 47.3% of the patients and actinic keratosis was present in 80.6% (Table 1). The mean time for the appearance of NMSC after the transplant was six years and nine months, ranging from two months to twenty-five years and three months, with the majority (39.8%) being diagnosed between one and five years after the transplant.
Clinical and epidemiological data of renal transplant recipients with and without non-melanoma skin cancer
| RTR with NMSC | RTR without NMSC | |||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Female | 22 | 23.7 | 47 | 39.2 |
| Male | 71 | 76.3 | 73 | 60.8 |
| Age (in years) | ||||
| <40 | 0 | 0 | 23 | 19.2 |
| 40 - 49 | 11 | 11.8 | 39 | 32.5 |
| 50 - 59 | 30 | 32.3 | 26 | 21.7 |
| 60 - 69 | 40 | 43 | 29 | 24.2 |
| ≥ 70 | 12 | 12.9 | 3 | 2.5 |
| Skin phototype | ||||
| I | 11 | 11.8 | 0 | 0 |
| II | 42 | 45.2 | 20 | 16.7 |
| III | 24 | 25.8 | 34 | 28.3 |
| IV | 11 | 11.8 | 47 | 39.2 |
| V | 0 | 0 | 8 | 6.7 |
| Unknown | 5 | 5.4 | 11 | 9.2 |
| NMSC type | ||||
| SCC | 45 | 48.4 | - | - |
| BCC | 14 | 15.1 | - | - |
| SCC and BCC | 34 | 36.6 | - | - |
| NMSC only on exposed areas | ||||
| Yes | 73 | 78.5 | - | - |
| No | 20 | 21.5 | - | - |
| Viral wart | ||||
| Yes | 44 | 47.3 | - | - |
| No | 49 | 52.7 | - | - |
| Actinic keratosis | ||||
| Yes | 75 | 80.6 | - | - |
| No | 18 | 19.4 | - | - |
| Time between renal transplant and the diagnosis of NMSC | ||||
| Less than 1 year | 6 | 6.5 | - | - |
| From 1 to 5 years | 37 | 39.8 | - | - |
| From 5 to 10 years | 32 | 34.4 | - | - |
| More than 10 years | 18 | 19.3 | - | - |
| CKD etiology | ||||
| Hypertension | 17 | 18.3 | 15 | 12.5 |
| Diabetes mellitus | 10 | 10.8 | 14 | 11.7 |
| Chronic glomerulonephritis | 18 | 19.4 | 13 | 10.8 |
| Chronic pyelonephritis | 2 | 2.2 | 1 | 0.8 |
| Urologic causes | 3 | 3.2 | 4 | 3.3 |
| Polycystic kidneys | 10 | 10.8 | 10 | 8.3 |
| Renal tuberculosis | 1 | 1.1 | 0 | 0 |
| Lupus nephritis | 0 | 0 | 4 | 3.3 |
| IgA nephropathy | 0 | 0 | 1 | 0.8 |
| Alport syndrome | 0 | 0 | 2 | 1.7 |
| Undetermined | 32 | 34.4 | 56 | 46.7 |
| Type of donor | ||||
| Deceased | 44 | 47.3 | 78 | 65 |
| Live | 49 | 52.7 | 42 | 35 |
RTR: renal transplant recipient, NMSC: non-melanoma skin cancer, SCC: squamous cell carcinoma, BCC: basal cell carcinoma, CKD: chronic kidney disease
Among the 120 RTRs without NMSC, 73 were male (60.8%). The mean age was 50 years, ranging from 23 to 72 years. The predominant phototype in the population analyzed was IV (39.2%), followed by III (28.3%). For most patients in this group, the etiology of the CKD was undetermined (46.7%). The etiologies identified were: hypertension in 12.5%; diabetes mellitus in 11.7%; chronic glomerulonephritis in 10.8%; polycystic kidney disease in 8.3%; urologic etiology in 3.3%; lupus nephritis in 3.3%; Alport syndrome in 1.7%; and IgA nephropathy in 0.8%. Renal transplant was from a deceased donor in 65.0% of the patients. Treatment regime for post-transplant immunosuppression also varied. In this sample, most patients used three drugs, while 11 patients used two drugs (Table 1).
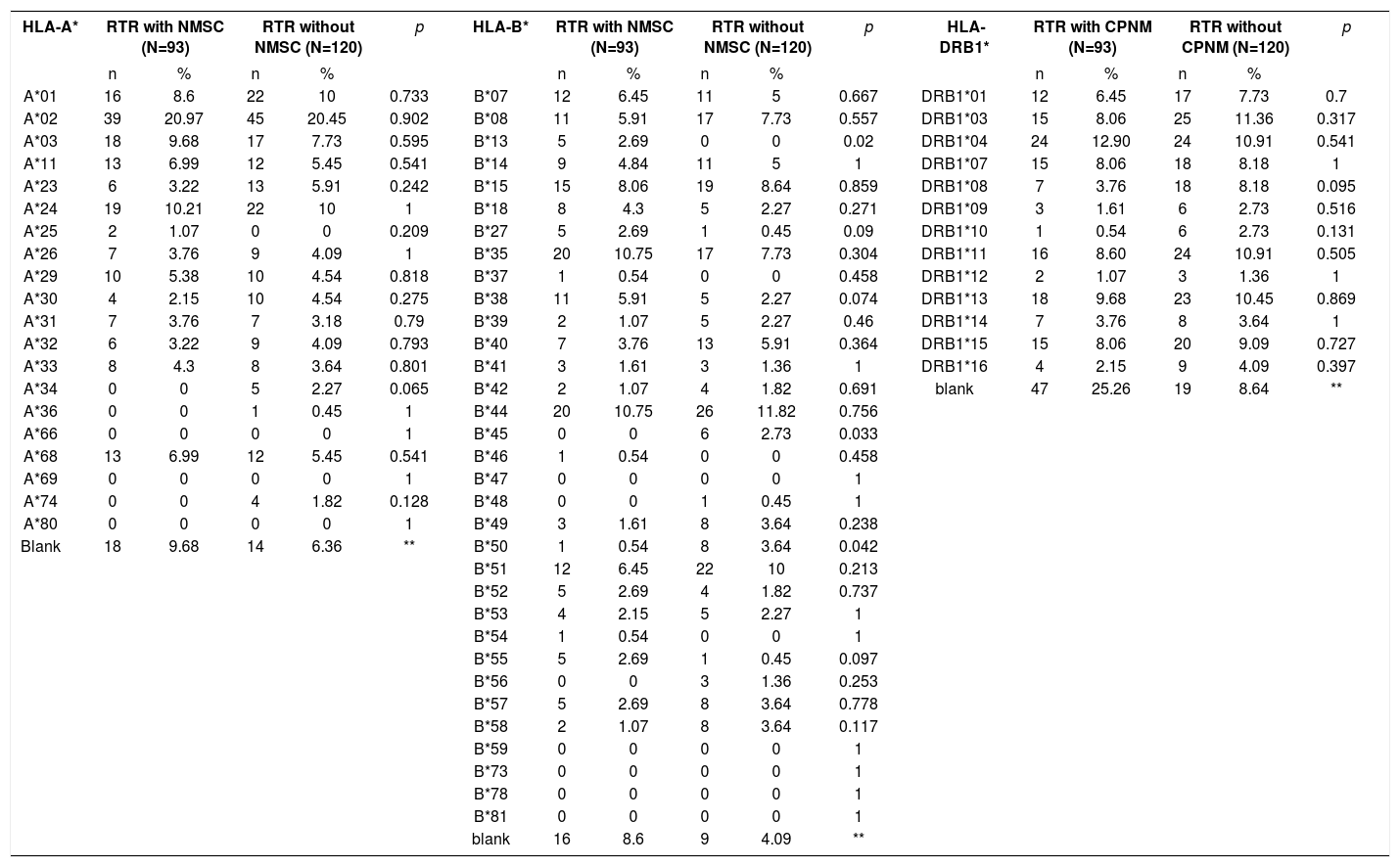
HLA-A, B and DRB1* frequencies in RTRs with and without NMSC are shown in table 2. Table 3 shows the significant results of the comparison between these groups. One positive association was found with HLA-B*13 (2.69% vs 0.00%, p=0.02, OR=7.29, 95%CI=0.86-61.07) in the group of RTRs with NMSC, suggesting they are more prone to developing NMSC. The alleles HLA-B*45 (0.00% vs 2.73%, p=0.033, OR=0.16, 95%CI= 0.02-1.35) and HLA-B*50 (0.54% vs 3.64%, p=0.042, OR=0.14, 95%CI=0.02-1.14) were more frequent in the group of RTRs without NMSC, linking those alleles to a lower probability of developing NMSC.
Frequencies of HLA-A, B and DRB1* alleles in RTR with NMSC and RTR without NMSC
| HLA-A* | RTR with NMSC (N=93) | RTR without NMSC (N=120) | p | HLA-B* | RTR with NMSC (N=93) | RTR without NMSC (N=120) | p | HLA-DRB1* | RTR with CPNM (N=93) | RTR without CPNM (N=120) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||||||
| A*01 | 16 | 8.6 | 22 | 10 | 0.733 | B*07 | 12 | 6.45 | 11 | 5 | 0.667 | DRB1*01 | 12 | 6.45 | 17 | 7.73 | 0.7 |
| A*02 | 39 | 20.97 | 45 | 20.45 | 0.902 | B*08 | 11 | 5.91 | 17 | 7.73 | 0.557 | DRB1*03 | 15 | 8.06 | 25 | 11.36 | 0.317 |
| A*03 | 18 | 9.68 | 17 | 7.73 | 0.595 | B*13 | 5 | 2.69 | 0 | 0 | 0.02 | DRB1*04 | 24 | 12.90 | 24 | 10.91 | 0.541 |
| A*11 | 13 | 6.99 | 12 | 5.45 | 0.541 | B*14 | 9 | 4.84 | 11 | 5 | 1 | DRB1*07 | 15 | 8.06 | 18 | 8.18 | 1 |
| A*23 | 6 | 3.22 | 13 | 5.91 | 0.242 | B*15 | 15 | 8.06 | 19 | 8.64 | 0.859 | DRB1*08 | 7 | 3.76 | 18 | 8.18 | 0.095 |
| A*24 | 19 | 10.21 | 22 | 10 | 1 | B*18 | 8 | 4.3 | 5 | 2.27 | 0.271 | DRB1*09 | 3 | 1.61 | 6 | 2.73 | 0.516 |
| A*25 | 2 | 1.07 | 0 | 0 | 0.209 | B*27 | 5 | 2.69 | 1 | 0.45 | 0.09 | DRB1*10 | 1 | 0.54 | 6 | 2.73 | 0.131 |
| A*26 | 7 | 3.76 | 9 | 4.09 | 1 | B*35 | 20 | 10.75 | 17 | 7.73 | 0.304 | DRB1*11 | 16 | 8.60 | 24 | 10.91 | 0.505 |
| A*29 | 10 | 5.38 | 10 | 4.54 | 0.818 | B*37 | 1 | 0.54 | 0 | 0 | 0.458 | DRB1*12 | 2 | 1.07 | 3 | 1.36 | 1 |
| A*30 | 4 | 2.15 | 10 | 4.54 | 0.275 | B*38 | 11 | 5.91 | 5 | 2.27 | 0.074 | DRB1*13 | 18 | 9.68 | 23 | 10.45 | 0.869 |
| A*31 | 7 | 3.76 | 7 | 3.18 | 0.79 | B*39 | 2 | 1.07 | 5 | 2.27 | 0.46 | DRB1*14 | 7 | 3.76 | 8 | 3.64 | 1 |
| A*32 | 6 | 3.22 | 9 | 4.09 | 0.793 | B*40 | 7 | 3.76 | 13 | 5.91 | 0.364 | DRB1*15 | 15 | 8.06 | 20 | 9.09 | 0.727 |
| A*33 | 8 | 4.3 | 8 | 3.64 | 0.801 | B*41 | 3 | 1.61 | 3 | 1.36 | 1 | DRB1*16 | 4 | 2.15 | 9 | 4.09 | 0.397 |
| A*34 | 0 | 0 | 5 | 2.27 | 0.065 | B*42 | 2 | 1.07 | 4 | 1.82 | 0.691 | blank | 47 | 25.26 | 19 | 8.64 | ** |
| A*36 | 0 | 0 | 1 | 0.45 | 1 | B*44 | 20 | 10.75 | 26 | 11.82 | 0.756 | ||||||
| A*66 | 0 | 0 | 0 | 0 | 1 | B*45 | 0 | 0 | 6 | 2.73 | 0.033 | ||||||
| A*68 | 13 | 6.99 | 12 | 5.45 | 0.541 | B*46 | 1 | 0.54 | 0 | 0 | 0.458 | ||||||
| A*69 | 0 | 0 | 0 | 0 | 1 | B*47 | 0 | 0 | 0 | 0 | 1 | ||||||
| A*74 | 0 | 0 | 4 | 1.82 | 0.128 | B*48 | 0 | 0 | 1 | 0.45 | 1 | ||||||
| A*80 | 0 | 0 | 0 | 0 | 1 | B*49 | 3 | 1.61 | 8 | 3.64 | 0.238 | ||||||
| Blank | 18 | 9.68 | 14 | 6.36 | ** | B*50 | 1 | 0.54 | 8 | 3.64 | 0.042 | ||||||
| B*51 | 12 | 6.45 | 22 | 10 | 0.213 | ||||||||||||
| B*52 | 5 | 2.69 | 4 | 1.82 | 0.737 | ||||||||||||
| B*53 | 4 | 2.15 | 5 | 2.27 | 1 | ||||||||||||
| B*54 | 1 | 0.54 | 0 | 0 | 1 | ||||||||||||
| B*55 | 5 | 2.69 | 1 | 0.45 | 0.097 | ||||||||||||
| B*56 | 0 | 0 | 3 | 1.36 | 0.253 | ||||||||||||
| B*57 | 5 | 2.69 | 8 | 3.64 | 0.778 | ||||||||||||
| B*58 | 2 | 1.07 | 8 | 3.64 | 0.117 | ||||||||||||
| B*59 | 0 | 0 | 0 | 0 | 1 | ||||||||||||
| B*73 | 0 | 0 | 0 | 0 | 1 | ||||||||||||
| B*78 | 0 | 0 | 0 | 0 | 1 | ||||||||||||
| B*81 | 0 | 0 | 0 | 0 | 1 | ||||||||||||
| blank | 16 | 8.6 | 9 | 4.09 | ** | ||||||||||||
RTR: renal transplant recipient, NMSC: non-melanoma skin cancer, N: number of individuals, n: number of alleles (2n), %: allelic frequency, p: Fisher’s exact test (p≤0.05), blank: homozygous or unidentified alleles, **: not calculated, bold font: significant association.
HLA alleles with statistically significant differences in RTR with NMSC and RTR without NMSC
| HLA Allele | RTR with NMSC (N=93) | RTR without NMSC (N=120) | p | OR | 95% CI | Association | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| B*13 | 5 | 2.69 | 0 | 0 | 0.02 | 7.29 | 0.86-61.07 | Higher probability of developing NMSC |
| B*45 | 0 | 0 | 6 | 2.73 | 0.033 | 0.16 | 0.02-1.35 | Lower probability of developing NMSC |
| B*50 | 1 | 0.54 | 8 | 3.64 | 0.042 | 0.14 | 0.02-1.14 | Lower probability of developing NMSC |
RTR: renal transplant recipient, NMSC: non-melanoma skin cancer, N: number of individuals, n: number of alleles (2n), %: allelic frequency, p: Fisher’s exact test (p≤0.05), OR: odds ratio, CI: confidence interval.
The lower risk of developing NMSC was attributed to HLA-A*34 (0.0% vs 17.4%, p=0.021, OR=0.12, 95%CI=0.01-1.03) in RTRs older than 50 years.
The analyses of the HLA alleles were also performed comparing skin phototypes and the presence of Bowen disease (intraepithelial SCC). No significant associations were found in these groups (data not shown).
Study limitationsHLA-A, B and DR alleles identified routinely in kidney and solid organ transplant use techniques of low and medium resolution that do not allow for the discrimination of specific alleles.
The limited number of patients included in this study is due to the availability of results in the files used for the research.
DiscussionSquamous cell carcinoma is the most common non-melanoma skin cancer in renal transplant recipients, followed by basal cell carcinoma. Among the transplant recipients, the frequency of SCC exceeds that of BCC with a ratio of 3:1; in the general population this index is the opposite.9–11 In the present study, we also observed a higher prevalence of SCC (85.0%), while 36.6% had SCC and BCC. BCC was found on its own in only 15.1% of the sample. The association with the occurrence of viral warts was not high (47.3%) but there was a high association with actinic keratosis (80.6%).
NMSCs are more aggressive in solid organ transplant recipients (SOTRs); one of the factors for this aggressive behavior could be the presence of angiogenic factors that stimulate tumor growth.12 In recent years, many studies show a higher incidence of skin cancer in RTRs, what could be explained by the increased survival of the patients, particularly due to the increased efficacy of immunosup-pressive drugs. Exposure to multiple carcinogenic agents such as ultraviolet radiation (UVR) and HPV (human papillomavirus) infection is also responsible for the increase in the incidence of NMSC. 3,11,13,14
Men are usually at a greater risk of developing NMSC, what was confirmed in our population, where 76.3% of patients were male. This could be attributed to many factors: men undergo renal transplants more frequently, have higher UVR exposure and are less prone to adhering to photoprotection. 3,9,15
In the present study, the predominant phototype in RTR with NMSC was II (45.2%), followed by III (25.8%), contrary to what is observed in the general immunocompetent population.16,17 Skin phototype I had a low prevalence (11.8%) and it might reflect the predominant skin phototype in the region of Brazil. The low prevalence (11.8%) of skin phototype IV was also observed, since these patients rarely develop NMSC. 15
The cause for kidney function loss was undetermined in the majority of cases. The most frequent causes were: hypertension, diabetes mellitus, chronic glomerulonephritis and polycystic kidney disease for both groups. The frequency of live donor was higher in RTR with NMSC (52.7%) but this was not statistically significant when compared to RTR without NMSC (35.0%).
Besides the factors already mentioned, other agents worked together promoting the increased risk of NMSC, including: individual genetic basis, age and sun exposure.3,6,18,19 The individual genetic basis that acts in the pathogenesis of skin cancer can be a protective or a susceptibility factor for the development of the disease. One of those determinants is HLA.20 The structural and functional changes in the HLA molecule can reduce the expression of tumor antigens. The absence of expression of co-stimulating molecules and the production of immunosuppressing cytokines are some of the possible mechanisms that help tumor cells escape immune surveillance, increasing the risk of developing tumor lesions such as cancer. 21
All donors and receptors are submitted to HLA typing before surgery or bone marrow transplant. The influence of HLA al-leles on the outcome of the renal transplant is considerable, since when the compatibility between the recipient and the donor increases, the risk of rejection is minimized.22
The mechanism by which the HLA system is involved in the development of NMSC is not yet entirely known. Some authors suggest the HLA alleles are associated to a higher or lower risk of skin cancer in solid organ transplant recipients (SOTR).8,23–26 HLA-DR homozygosity is associated to skin cancer in general, while HLA-DR7 was suggested as a protective factor for the development of skin cancer in general, squamous cell carcinoma and Bowen disease.23,24,25 In Brazil, HLA-DR1 appeared as a risk factor for NMSC, BCC in particular, even though this allele is not related to skin cancer in RTR in the European population.8,27 Recently, Cegielska et al showed that the HLA-DR15 allele is a susceptibility factor for NMSC, and that HLA-B18 is related to SCC.26
HLA-A1 and HLA-A11 alleles were cited as protective factors for Bowen disease in Australian patients.25 It was suggested that HLA-A11 is a protective allele for HPV-related cervical cancer. Bavinck et al showed that, in patients living in the Netherlands, HLA-A11 is a protective for skin cancer in general.23 Surprisingly, when the same HLA-A11 was studied in Queensland, this allele was related to a higher occurrence of skin cancer.24 The authors raised the hypothesis that UVR-induced photoantigens in Australian patients can compensate the risk factors induced by HPV infection in a more temperate climate. 24 It was demonstrated that HLA-B27 is a susceptibility factor for NMSC, particularly BCC.23 Ingvar et al did not find any association between HLA typing and the risk for SCC in SOTRs.28
In the immunocompetent population, the incidence of BCC was associated to HLA-DR1 and HLA-DR7, while HLA-DR4 was protective for the development of the disease, but there still are no solid evidences. Besides, HLA-A11 and HLA-B27 alleles are associated to the development of NMSC.27,29
The present study, conducted in a region with a tropical climate, with a historically mixed population, showed that the risk for developing NMSC among individuals undergoing a transplant was attributed to HLA-B*13, while the alleles HLA-B*45 and B*50 are associated to a lower probability of developing NMSC. The presence of HLA-A*34 was attributed to a lower risk for developing NMSC in RTRs older than 50 years of age. Epidemiological and clinical data did not show any correlation with HLA alleles.
ConclusionThe involvement of HLA alleles in the development of NMSC in RTRs was confirmed in this study, although with different alleles than those previously described in the literature. This fact can be attributed to the variation in the genetic background and geographical regions in which the studies were conducted, even in Brazil. However, the patients that have the HLA-B*13 allele require a long-term follow-up due to the higher probability (risk) of developing skin cancer after the transplant.
Work conducted at the Department of Dermatology, Universidade Federal de São Paulo, São Paulo (SP), Brazil.
Financial Support: FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) through scholarship granted to the first author.
Conflict of interest: None.